Metals in the periodic table
All elements in Dmitri Mendeleev's periodic table are divided into two large groups: metals and non-metals. The first is the most numerous. Most elements are metals (blue). Nonmetals in the table are shown on a yellow background. There is also a group of elements that are classified as metalloids (red). All metals are grouped on the left side of the table. Notice that hydrogen is grouped with metals in the upper left corner. Despite this, it is considered non-metallic. However, some scientists theorize that there may be metallic hydrogen in the core of the planet Jupiter.
Classification
Of the 118 chemical elements discovered so far, metals include:
6 elements in the alkali metal group: Li, Na, K, Rb, Cs, Fr;
4 in the group of alkaline earth metals: Ca, Sr, Ba, Ra;
and also outside certain groups beryllium and magnesium;
40 in the transition metal group:
— Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn; — Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd; — La, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg; — Ac, Rf, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn;
7 in the group of light metals: Al, Ga, In, Sn, Tl, Pb, Bi;
7 in the group of semimetals[1]: B, Si, Ge, As, Sb, Te, Po;
14 in the lanthanide + lanthanum (La) group: Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu;
14 in the group actinides (physical properties have not been studied for all elements) + actinium (Ac): Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr.
Hydrogen can also have metallic properties[2][3].
Thus, 94 of all discovered elements may be metals; all others are non-metals.
In astrophysics, the term "metal" can have a different meaning and refer to all chemical elements heavier than helium (see metallicity)
.
In addition, in physics, metals, as conductors, are contrasted with semiconductors and dielectrics (see also Semimetal (spintronics)
)[4].
Some groups/families of metals
Osmium

Aluminum

Barium
- Alkaline:
- Lithium
- Sodium
- Potassium
- Rubidium
- Cesium
- France
- Alkaline earth:
- Calcium
- Strontium
- Barium
- Radium
- Others (which are often not quite correctly classified as alkaline earth):
- Beryllium
- Magnesium
- Transitional:
- Uranus
- Titanium
- Iron
- Platinum
- Copper
- Zinc
- Gold
- Silver
- Palladium
- Mercury
- Nickel
- Cobalt
- Tungsten
- Post-transition:
- Lightweight: Aluminum
- Gallium
- Lead
- Tin
- Heavy:
- Lead
- Mercury
- Copper
- Cadmium
- Cobalt
- Refractory
- Platinum group metals
- Colored
- Noble
- Coin
Amorphous metals
Main article: Amorphous metals
Metal bondage
Many of the wonderful and beneficial qualities of an element come from the way its atoms bond together. In this case, certain connections arise. The metallic interaction of atoms results in the creation of metallic structures. Every instance of this element in everyday life, from a car to coins in a pocket, involves a metal connection.

During this process, the metal atoms share their outer electrons evenly with each other. Electrons flowing between positively charged ions easily transfer heat and electricity, making these elements such good conductors of heat and electricity. Copper wires are used for power supply.

Metal structure
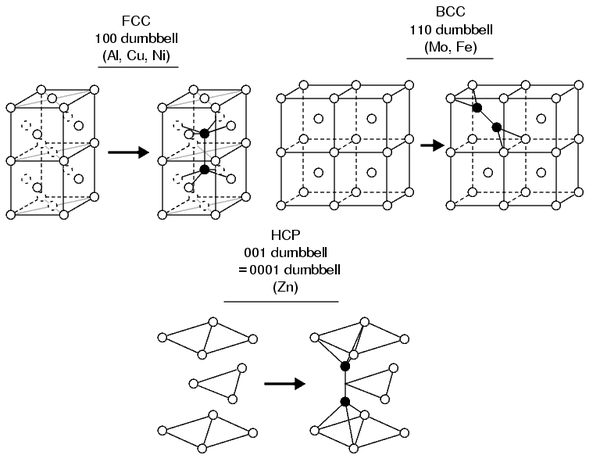
Crystal structure of alloys
See also: Metallurgy
No metal can be prepared in an absolutely pure state. Technically “pure” metals can contain up to several percent impurities, and if these impurities are elements with low atomic weight (for example, carbon, nitrogen or oxygen), then in terms of atomic percentages the content of these impurities can be very large. The first small amounts of impurities in the metal usually enter the crystal in the form of a solid solution. There are two main types of solid solutions:
- first, when the impurity atoms are much smaller than the solvent metal atoms, the dissolved atoms are located in the solvent lattice along interstices, or “voids.” The formation of such solid solutions—interstitial solid solutions—is almost always accompanied by expansion of the solvent lattice, and in the vicinity of each dissolved atom there is a local distortion of the lattice;
- second, when the atoms of the impurity and the solvent have approximately the same sizes, a substitution solid solution is formed, in which atoms of the dissolved element replace atoms of the solvent, so that atoms of both types occupy places at the sites of the common lattice. In such cases, there is also a distorted region around each dissolved atom, and whether the lattice will expand or contract depends on the relative sizes of the solvent and solute atoms [11].
For most metals, the most important elements forming interstitial solid solutions are hydrogen, boron, carbon, nitrogen and oxygen. The presence of dislocations always leads to the appearance of abnormally large or small interatomic distances. In the presence of impurities, each dislocation is surrounded by an “atmosphere” of impurity atoms. Impurity a dislocations, because as a result of the movement of dislocations a new configuration with increased energy will be formed. The boundaries between crystals are also regions with anomalous interatomic distances and, therefore, also dissolve impurity atoms more easily than undistorted regions of crystals.
As the impurity content increases, dissolved atoms enter the bulk of the crystal, but there is still an excess of impurities along the grain boundaries and around dislocations. When the impurity exceeds the solubility limit, a new phase appears, which can be either a solute, an intermediate phase, or a compound. In such cases, the boundaries between phases can be of two kinds. In general, the crystal structure of impurity particles is too different from the structure of the solvent metal, so the lattices of the two phases cannot transform into one another, forming a continuous structure. In such cases, layers with an irregular (distorted) structure are formed at the phase boundaries. The formation of boundaries is associated with the appearance of free surface energy, but the deformation energy of the solvent lattice is relatively small. In such cases, they say that these particles are released incoherently.
In some cases, the interatomic distances and the crystal structure of the solvent metal and impurity particles are such that some planes can be connected to each other, forming a continuous structure. Then they say that the particles of the second phase are released coherently and, since the conjugation of the lattices is never absolutely precise, a highly stressed region is formed around the boundary. In cases where the deformation energy is too high for this, neighboring crystals can come into contact in such a way that regions of elastic deformation appear in the boundary layers, and dislocations appear at the interface itself. In such cases, they say that the particles are released semi-coherently[12].
As the temperature increases, due to an increase in the amplitude of atomic vibrations, a defect in the crystal lattice, which is called a vacancy or “hole,” can form. Diffusion of vacancies is one of the mechanisms of dislocation formation[13].
As a rule, metal crystallization occurs by supercooling with the formation of a dendritic structure. As they grow, dendritic crystals come into contact, and various structural defects are formed. In most cases, the metal hardens so that the first batch of crystals contains fewer impurities than subsequent ones. Therefore, as a rule, impurities are concentrated at grain boundaries, forming stable structures [14].
Metal reactions
Reactivity refers to the tendency of an element to react with chemicals in its environment. It can be different. Some metals, such as potassium and sodium (in columns 1 and 2 of the periodic table), react easily with many different chemicals and are rarely found in their pure, elemental form. Both usually exist only in compounds (bonded to one or more other elements) or as ions (a charged version of their elemental form).

On the other hand, there are other metals, they are also called jewelry. Gold, silver and platinum are not very reactive and are usually found in pure form. These metals lose electrons more easily than nonmetals, but not as easily as reactive metals such as sodium. Platinum is relatively unreactive and very resistant to reactions with oxygen.
Mendeleev's periodic table
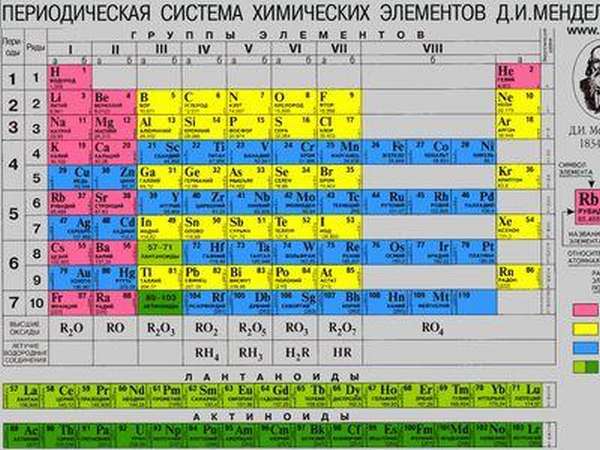
The periodic table of chemical elements was compiled by D.I. Mendeleev in the second half of the 19th century. What is it and what is it for? It unites all chemical elements in order of increasing atomic weight, and they are all arranged in such a way that their properties change in a periodic manner.
Mendeleev's periodic system in chemistry brought together into a single system all existing elements, previously considered simply individual substances.
Based on its study, new chemical substances were predicted and subsequently synthesized. The significance of this discovery for science cannot be overestimated ; it was significantly ahead of its time and gave impetus to the development of chemistry for many decades.
There are three most common table options, which are conventionally called “short”, “long” and “extra long ”. The long table is considered the main one; it is officially approved. The difference between them is the arrangement of elements and the length of periods.
What is a period
The system contains 7 periods . They are presented graphically as horizontal lines. In this case, a period can have one or two lines, called rows. Each subsequent element differs from the previous one by increasing the nuclear charge (number of electrons) by one.
To keep it simple, a period is a horizontal row of the periodic table. Each of them begins with metal and ends with an inert gas. Actually, this creates periodicity - the properties of elements change within one period, repeating again in the next. The first, second and third periods are incomplete, they are called small and contain 2, 8 and 8 elements, respectively. The rest are complete, they have 18 elements each.
What is a group
A group is a vertical column containing elements with the same electronic structure or, more simply, with the same highest valence. The officially approved long table contains 18 groups, which begin with alkali metals and end with noble gases.
Each group has its own name, making it easier to search or classify elements. Metallic properties are enhanced, regardless of the element, from top to bottom. This is due to an increase in the number of atomic orbits; the more there are, the weaker the electronic bonds, which makes the crystal lattice more pronounced.
Element properties
When you learned the alphabet in elementary school, you discovered that all letters have their own unique set of properties. For example, some had straight lines, some had curves, and others had both types of lines. The same can be said about the elements. Each of them has a unique set of physical and chemical properties. Physical properties are qualities inherent in certain substances. Shiny or not, how well it conducts heat and electricity, at what temperature it melts, how dense it is.

Chemical properties include those qualities that are observed when exposed to oxygen if they were to burn (how difficult it would be for them to retain their electrons during a chemical reaction). Different elements may have common properties. For example, iron and copper are both elements that conduct electricity. However, they do not have the same properties. For example, when iron is exposed to humid air, it becomes rusty, but when copper is exposed to the same conditions, it acquires a specific green patina. That's why the Statue of Liberty is green and not rusty. It is made of copper, not iron).

Interaction of acids with metals
Metals react differently with acids. Metals in the electrochemical activity series of metals (ERAM) up to hydrogen interact with almost all acids.
Interaction of non-oxidizing acids with metals in the electrical activity series of metals up to hydrogen
A substitution reaction occurs, which is also redox:
M g + 2 HC l = M g C l 2 + H 2 ↑ {\displaystyle {\mathsf {Mg+2HCl=MgCl_{2}+H_{2}\uparrow }}} 2 A l + 2 H 3 PO 4 = 2 A l PO 4 + 3 H 2 ↑ {\displaystyle {\mathsf {2Al+2H_{3}PO_{4}=2AlPO_{4}+3H_{2}\uparrow }}}
Reaction of concentrated sulfuric acid H2SO4 with metals
Oxidizing acids can also interact with metals located in the ERAM after hydrogen:
C u + 2 H 2 SO 4 = C u SO 4 + SO 2 ↑ + 2 H 2 O {\displaystyle {\mathsf {Cu+2H_{2}SO_{4}=CuSO_{4}+SO_{2}\ uparrow +2H_{2}O}}}
A highly dilute acid reacts with a metal according to the classical scheme:
M g + H 2 SO 4 = M g SO 4 + H 2 ↑ {\displaystyle {\mathsf {Mg+H_{2}SO_{4}=MgSO_{4}+H_{2}\uparrow }}}
As the acid concentration increases, various products are formed:
M g + 2 H 2 SO 4 = M g SO 4 + SO 2 ↑ + 2 H 2 O {\displaystyle {\mathsf {Mg+2H_{2}SO_{4}=MgSO_{4}+SO_{2}\ uparrow +2H_{2}O}}} 3 M g + 4 H 2 SO 4 = 3 M g SO 4 + S ↓ + 4 H 2 O {\displaystyle {\mathsf {3Mg+4H_{2}SO_{4} =3MgSO_{4}+S\downarrow +4H_{2}O}}} 4 M g + 5 H 2 SO 4 = 4 M g SO 4 + H 2 S ↑ + 4 H 2 O {\displaystyle {\mathsf { 4Mg+5H_{2}SO_{4}=4MgSO_{4}+H_{2}S\uparrow +4H_{2}O}}}
Reactions for nitric acid (HNO3)

When interacting with active metals, there are even more reaction options:
Z n + 4 HNO 3 ( 60 % ) = Z n ( NO 3 ) 2 + 2 NO 2 ↑ + 2 H 2 O {\displaystyle {\mathsf {Zn+4HNO_{3}(60\%)=Zn(NO_ {3})_{2}+2NO_{2}\uparrow +2H_{2}O}}} 3 Z n + 8 HNO 3 ( 30%) = 3 Z n ( NO 3 ) 2 + 2 NO ↑ + 4 H 2 O {\displaystyle {\mathsf {3Zn+8HNO_{3}(30\%)=3Zn(NO_{3})_{2}+2NO\uparrow +4H_{2}O}}} 4 Z n + 10 HNO 3 ( 20 % ) = 4 Z n ( NO 3 ) 2 + N 2 O ↑ + 5 H 2 O {\displaystyle {\mathsf {4Zn+10HNO_{3}(20\%)=4Zn(NO_{3 })_{2}+N_{2}O\uparrow +5H_{2}O}}} 5 Z n + 12 HNO 3 (10%) = 5 Z n ( NO 3 ) 2 + N 2 ↑ + 6 H 2 O {\displaystyle {\mathsf {5Zn+12HNO_{3}(10\%)=5Zn(NO_{3})_{2}+N_{2}\uparrow +6H_{2}O}}} 4 Z n + 10 HNO 3 ( 3 % ) = 4 Z n ( NO 3 ) 2 + NH 4 NO 3 + 3 H 2 O {\displaystyle {\mathsf {4Zn+10HNO_{3}(3\%)=4Zn(NO_ {3})_{2}+NH_{4}NO_{3}+3H_{2}O}}}
Alloying
Main article: Alloying (metallurgy)
Alloying is the introduction of additional elements into the melt that modify the mechanical, physical and chemical properties of the base material.
Organization of elements: metals and nonmetals
The fact that elements have some common and unique properties allows them to be sorted into a nice, neat diagram called the periodic table. It organizes elements based on their atomic number and properties. So, in the periodic table we find elements grouped together that have common properties. Iron and copper are close to each other, both are metals. Iron is represented by the symbol "Fe" and copper is represented by the symbol "Cu".

Most elements in the periodic table are metals, and they tend to be on the left side of the table. They are grouped together because they have certain physical and chemical properties. For example, metals are dense, shiny, good conductors of heat and electricity, and they easily lose electrons in chemical reactions. In contrast, nonmetals have the opposite properties. They are not dense, do not conduct heat or electricity, and tend to gain electrons rather than give them away. When we look at the periodic table, we see that most nonmetals are grouped on the right. These are elements such as helium, carbon, nitrogen and oxygen.

Application
People have learned to use metals in almost all areas of their lives. They are used to make construction materials, wires, electrical equipment, and dishes. Unstable radioactive elements such as uranium, californium, and polonium have found use in nuclear energy and weapons production.
Lightweight and durable metals are used in space technology and the automotive industry. Various elements are used in pharmaceuticals, food, and textile industries. They are used to make jewelry, household items, as well as medicines and medical instruments. Lithium, for example, is used as an antidepressant, gold is included in drugs against arthritis and tuberculosis. Titanium and tantalum are used in surgery for prosthetics and replacement of damaged parts of the body.
What are heavy metals?
The list of metals is quite numerous. Some of them can accumulate in the body without causing harm to it, such as natural strontium (formula Sr), which is an analogue of calcium, since it is productively deposited in bone tissue. Which of them are called heavy and why? Let's look at four examples: lead, copper, mercury and arsenic.
Where are these elements located and how do they affect the environment and human health? Heavy metals are metallic, naturally occurring compounds that have very high densities compared to other metals—at least five times the density of water. They are toxic to humans. Even small doses can lead to serious consequences.

- Lead. It is a heavy metal that is toxic to people, especially children. Poisoning with this substance can lead to neurological problems. Although it was once very attractive due to its flexibility, high density and ability to absorb harmful radiation, lead has been phased out in many ways. This soft, silvery metal, which is found on Earth, is dangerous to humans and accumulates in the body over time. The worst thing is that you cannot get rid of it. It sits there, accumulates and gradually poisons the body. Lead is toxic to the nervous system and can cause severe brain damage in children. It was widely used in the 1800s to create makeup and was used as an ingredient in hair dye until 1978. Today, lead is used primarily in large batteries, as shields for X-rays, or as insulation for radioactive material.
- Copper. It is a reddish-brown heavy metal that has many uses. Copper is still one of the best conductors of electricity and heat, and many electrical wires are made from this metal and covered with plastic. Coins, mostly small change, are also made from this element of the periodic table. Acute copper poisoning is rare, but like lead, it can accumulate in tissues, eventually leading to toxicity. People who are exposed to large amounts of copper or copper dust are also at risk.
- Mercury. This metal is toxic in any form and can even be absorbed by the skin. Its uniqueness lies in the fact that it is liquid at room temperature and is sometimes called "fast silver". It can be seen in a thermometer because as a liquid it absorbs heat, changing volume with even the slightest difference in temperature. This allows the mercury to rise or fall in the glass tube. Because this substance is a potent neurotoxin, many companies are switching to red-colored alcohol thermometers.
- Arsenic. From Roman times until the Victorian era, arsenic was considered the “king of poisons” as well as the “poison of kings.” History is riddled with countless examples of both royalty and common people committing murder for personal gain using arsenic compounds that had no smell, color or taste. Despite all the negative effects, this metalloid also has its own areas of application, even in medicine. For example, arsenic trioxide is a very effective drug used to treat people with acute promyelocytic leukemia.
Metal production
Ore preparation
Main articles: Ore
,
Mining
,
Ore beneficiation
,
Metallurgy
,
Metallurgy
Metals are extracted from the earth during the mining process. The mined ores serve as a relatively rich source of essential elements. To determine the location of ores in the earth's crust, special search methods are used, including exploration and study of ore deposits. Ore deposits are developed by open-pit or quarry methods and underground or mine methods. Sometimes a combined (open-underground) method of developing ore deposits is used.
After ores are extracted, they are usually subjected to beneficiation. In this case, one or more useful components are isolated from the initial mineral raw materials - ore concentrate(s), middlings and waste tailings. In enrichment processes, the differences between minerals of the useful component and waste rock in density, magnetic susceptibility, wettability, electrical conductivity, size, grain shape, chemical properties, etc. are used.
Working with ore
Metals are usually extracted from mined and enriched ore using chemical or electrolytic reduction. In pyrometallurgy, high temperatures are used to convert ore into metal raw materials; in hydrometallurgy, aqueous chemistry is used for the same purposes. The methods used depend on the type of metal and the type of contamination.
When a metal ore is an ionic compound of a metal and a non-metal, it is usually subjected to smelting—heating with a reducing agent—to extract the pure metal. Many common metals such as iron, copper, and tin are smelted using carbon as a reducing agent. Some metals, such as aluminum and sodium, do not have any economically feasible reducing agent and are recovered using electrolysis.[8][9]
Sulfide ores are not directly refined into pure metal, but are roasted in air to convert them into oxides.
What is a precious metal?
A precious metal is a metal that can be rare or difficult to obtain and is also economically very valuable. What is the list of metals that are precious? There are three in total:
- Platinum. Despite its refractory nature, it is used in jewelry, electronics, automobiles, chemical processes, and even medicine.
- Gold. This precious metal is used to make jewelry and gold coins. However, it has many other uses. It is used in medicine, manufacturing and laboratory equipment.
- Silver. This noble silvery-white metal is highly malleable. in its pure form it is quite heavy, it is lighter than lead, but heavier than copper.
History of the development of ideas about metals
See also: History of iron production and use
Man's acquaintance with metals began with gold, silver and copper, that is, with metals found in a free state on the earth's surface; subsequently they were joined by metals that are widely distributed in nature and are easily isolated from their compounds: tin, lead, iron and mercury. These seven metals were familiar to mankind in ancient times. Among the ancient Egyptian artifacts there are gold and copper items, which, according to some data, date back to an era 3000-4000 years removed from the present era. e.
To the seven known metals, only in the Middle Ages were added zinc, bismuth, antimony and at the beginning of the 18th century arsenic. From the middle of the 18th century, the number of discovered metals increased rapidly and by the beginning of the 20th century it reached 65, and by the beginning of the 21st century - up to 96.
None of the chemical industries contributed as much to the development of chemical knowledge as the processes associated with the production and processing of metals; The most important moments in the history of chemistry are connected with their history. The properties of metals are so characteristic that already in the earliest era gold, silver, copper, lead, tin, iron and mercury constituted one natural group of homogeneous substances, and the concept of “metal” refers to the most ancient chemical concepts. However, views on their nature in a more or less definite form appeared only in the Middle Ages among alchemists. True, Aristotle’s ideas about nature: the formation of everything that exists from four elements (fire, earth, water and air) thereby already indicated the complexity of metals; but these ideas were too vague and abstract. For alchemists, the concept of the complexity of metals and, as a result of this, belief in the ability to transform some metals into others, to create them artificially, is the main concept of their worldview.
Only Lavoisier clarified the role of air in combustion and showed that the gain in the weight of metals during firing comes from the addition of oxygen from the air to the metals, and thus established that the act of combustion of metals is not disintegration into elements, but, on the contrary, an act of connection, a question of the complexity of metals was resolved negatively. Metals were classified as simple chemical elements, due to Lavoisier's basic idea that simple bodies are those from which it was not possible to isolate other bodies. With the creation of the periodic system of chemical elements by Mendeleev, the metal elements took their rightful place in it.
Metals: types and properties
Most elements can be considered metals. They are grouped in the middle on the left side of the table. Metals are alkali, alkaline earth, transition, lanthanide and actinide.

They all have several common properties, these are:
- solid at room temperature (except mercury);
- usually shiny;
- with a high melting point;
- good conductor of heat and electricity;
- with low ionization ability;
- with low electronegativity;
- malleable (able to take a given shape);
- plastic (can be pulled into wire);
- with high density;
- a substance that loses electrons in reactions.
List of metals known to science
- lithium;
- beryllium;
- sodium;
- magnesium;
- aluminum;
- potassium;
- calcium;
- scandium;
- titanium;
- vanadium;
- chromium;
- manganese;
- iron;
- cobalt;
- nickel;
- copper;
- zinc;
- gallium;
- rubidium;
- strontium;
- yttrium;
- zirconium;
- niobium;
- molybdenum;
- technetium;
- ruthenium;
- rhodium;
- palladium;
- silver;
- cadmium;
- indium;
- copernicium;
- cesium;
- barium;
- tin;
- iron;
- bismuth;
- lead;
- mercury;
- tungsten;
- gold;
- platinum;
- osmium;
- hafnium;
- germanium;
- iridium;
- niobium;
- rhenium;
- antimony;
- thallium;
- tantalum;
- francs;
- livermorium.

In total, about 105 chemical elements are known, most of which are metals. The latter are a very common element in nature, which is found both in pure form and as part of various compounds.

Metals lie in the depths of the earth; they can be found in various bodies of water, in the bodies of animals and humans, in plants and even in the atmosphere. In the periodic table they are arranged starting with lithium (a metal with the formula Li) and ending with livermorium (Lv). The table continues to be replenished with new elements, and these are mainly metals.
Being in nature
Metals are widespread on our planet. They are found in seawater in the form of salts and compounds. Most of all, it is full of magnesium (0.12%) and calcium (1.05%). The most common metal in the earth's crust is aluminum. It makes up approximately 8% of its total mass. It also contains a lot of iron (4.1%), calcium (4%), sodium (2.3%), magnesium (2.3%), potassium (2.1%).

But metals are present not only in the external environment. They are present in any living organism, responsible for many vital functions. The human body contains about 3% metals. Iron in the blood helps hemoglobins attach oxygen and exchange with carbon dioxide. Magnesium is found in muscles and the nervous system. It is involved in protein synthesis, is responsible for muscle relaxation, and inhibits the excitation of nerve endings.
The most necessary for us: magnesium, iron, sodium, calcium, potassium, zinc, copper, cobalt, manganese and molybdenum. Metals are found in bones, in the brain, and in the tissues of other organs. We get them through water and food, and constantly need to replenish their supplies. With a deficiency of these elements, the body does not function properly, however, their excess is also not beneficial.









