Since time immemorial, people have been actively using various metals. After studying their properties, the substances took their rightful place in the table of the famous D. Mendeleev. Scientists are still arguing about the question of which metal should be given the title of the heaviest and densest in the world. There are two elements in the balance on the periodic table – iridium and osmium. Why they are interesting, read on.
Tantalum - 16.67 g/cm³

Extremely refractory (melting point 3017 °C), tantalum successfully replaces platinum in many cases.
It is used in jewelry - watch cases, bracelets and other jewelry are made from it. This is facilitated by the high hardness of the metal. In addition, it is cheaper than platinum, although more expensive than silver.
Its compounds replace platinum as catalysts in the chemical industry. In glassmaking, the addition of this metal to the melt makes it possible to obtain glasses used for the production of small binoculars and lightweight glasses. And tantalum is absolutely irreplaceable in the production of radio electronics.
Vivid characteristics of the densest metals
The substances obtained experimentally are powders that are quite difficult to process; forging metals requires very high temperatures. The most common form of the combination of iridium and osmium is the alloy of osmic iridium, which is mined in platinum deposits and gold strata.
The most common places where iridium is found are meteorites rich in iron. Native osmium cannot be found in the natural world, only in collaboration with iridium and other components of the platinum group. Deposits often contain sulfur and arsenic compounds.
Features of the heaviest and most expensive metal in the world
Among the elements of Mendeleev's periodic table, osmium is considered the most expensive. The silvery metal with a bluish tint belongs to the platinum group of noble chemical compounds. The densest, but very brittle metal does not lose its shine under the influence of high temperatures.

Characteristics
- Element #76 Osmium has an atomic mass of 190.23 amu;
- A substance molten at a temperature of 3033°C will boil at 5012°C.
- The heaviest material has a density of 22.62 g/cm³;
- The structure of the crystal lattice has a hexagonal shape.
Despite the amazingly cold shine of silver tint, osmium is not suitable for the production of jewelry due to its high toxicity. Melting the jewelry would require a temperature similar to the surface of the Sun, since the densest metal in the world is destroyed by mechanical stress.
Turning into powder, osmium interacts with oxygen, reacts to sulfur, phosphorus, selenium; the reaction of the substance to aqua regia is very slow. Osmium does not have magnetism; alloys tend to oxidize and form cluster compounds.

Where is it used?
The heaviest and incredibly dense metal has high wear resistance, so adding it to alloys significantly increases their strength. The use of osmium is mainly associated with the chemical industry. In addition, it is used for the following needs:
- manufacturing containers intended for storing nuclear fusion waste;
- for the needs of rocket science, weapons production (warheads);
- in the watch industry for the manufacture of movements of branded models;
- for the manufacture of surgical implants, parts of pacemakers.
Interestingly, the densest metal is considered the only element in the world that is not subject to the aggression of the “hellish” mixture of acids (nitric and hydrochloric). Aluminum combined with osmium becomes so ductile that it can be pulled without breaking.
Secrets of the world's rarest and densest metal
The fact that iridium belongs to the platinum group gives it the property of immunity to treatment with acids and their mixtures. In the world, iridium is obtained from anode sludge during copper-nickel production. After treating the sludge with aqua regia, the resulting precipitate is calcined, resulting in the extraction of iridium.

Characteristics
The hardest silver-white metal has the following group of properties:
- periodic table element Iridium No. 77 has an atomic mass of 192.22 amu;
- a substance melted at a temperature of 2466°C will boil at 4428°C;
- density of molten iridium – within 19.39 g/cm³;
- element density at room temperature – 22.7 g/cm³;
- The iridium crystal lattice is associated with a face-centered cube.
Heavy iridium does not change under the influence of normal air temperature. The result of calcination under the influence of heat at certain temperatures is the formation of multivalent compounds. The powder of fresh sediment of iridium black can be partially dissolved with aqua regia, as well as with a chlorine solution.

Application area
Although Iridium is a precious metal, it is rarely used for jewelry. The element, which is difficult to process, is in great demand in the construction of roads and the production of automobile parts. Alloys with the densest metal that is not susceptible to oxidation are used for the following purposes:
- manufacturing crucibles for laboratory experiments;
- production of special mouthpieces for glass blowers;
- covering the tips of pens and ballpoint pens;
- production of durable spark plugs for cars;
Alloys with iridium isotopes are used in welding production, in instrument making, and for growing crystals as part of laser technology. The use of the heaviest metal made it possible to carry out laser vision correction, crushing kidney stones and other medical procedures.
Although Iridium is non-toxic and not dangerous to biological organisms, its dangerous isotope, hexafluoride, can be found in the natural environment. Inhalation of toxic vapors leads to instant suffocation and death.
Uranium - 19.05 g/cm³

The name of the planet of the solar system came from the name of this element, and not vice versa, as many believe.
It is a very heavy, flexible and malleable metal. Capable of self-ignition. There is a lot of it both in the earth's crust and in sea water.
Thanks to uranium, invisible rays were accidentally discovered at the end of the 19th century (today the phenomenon of the emission of invisible rays by some natural substances is called radioactivity).
Natural uranium oxides have been used since ancient times in the manufacture of glazes for ceramic products. Nowadays, compounds of this metal are also used to create yellow paint.
general information
For centuries, people have been studying the beneficial properties of the most common metals on the planet. Science stores the most information about gold, silver and copper. Over time, humanity became acquainted with iron and lighter metals - tin and lead. In the world of the Middle Ages, people actively used arsenic, and diseases were treated with mercury.
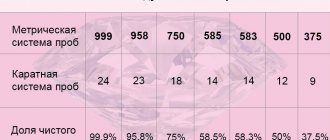
Thanks to rapid progress, today the heaviest and densest metals are considered not just one element of the table, but two at once. At number 76 is osmium (Os), and at number 77 is iridium (Ir), the substances have the following density indicators:
- osmium is heavy, due to its density of 22.62 g/cm³;
- iridium is not much lighter - 22.53 g/cm³.
Density is one of the physical properties of metals; it is the ratio of the mass of a substance to its volume. Theoretical calculations of the density of both elements have some errors, so both metals are today considered to be the heaviest.
For clarity, you can compare the weight of an ordinary cork with the weight of a cork made of the heaviest metal in the world. To balance the scales with a stopper made of osmium or iridium, you will need more than a hundred ordinary stoppers.
Tungsten - 19.29 g/cm³

Absolute champion in refractoriness. It boils at a temperature of 5555 °C (the same in the photosphere of the Sun).
The word tungsten means “devouring tin like a wolf devouring a sheep.” This name did not appear by chance. Tungsten, being among the tin ores, interfered with the smelting of tin.
Used to create wedding rings. Its strength symbolizes the stability of personal relationships. In addition, polished tungsten cannot be scratched by anything.
It is used in the production of filaments in various lighting devices.
Gold - 19.29 g/cm³

The gold content in the ground is very low, although there are many deposits rich in it. There is even a little gold in water - in every cubic meter there is at least five micrograms of gold.
Under normal conditions, it does not oxidize and does not interact with most acids, therefore it is considered a noble metal.
Gold easily transmits heat and electricity, making it indispensable in radio electronics.
Density of matter beyond planet Earth
Osmium is undoubtedly the leader of the heaviest elements on our planet. But if we turn our gaze into space, then our attention will reveal many substances heavier than our “king” of heavy elements.
The fact is that in the Universe there are conditions somewhat different than on Earth. The gravity of a number of space objects is so great that the matter becomes incredibly dense.
If we consider the structure of the atom, we will find that the distances in the interatomic world are somewhat reminiscent of the space we see. Where planets, stars and other cosmic bodies are at a fairly large distance. The rest is occupied by emptiness. This is exactly the structure that atoms have, and with strong gravity this distance decreases quite significantly. Up to the “pressing” of some elementary particles into others.
Plutonium - 19.80 g/cm³
The first artificial chemical element, whose production began on an industrial scale almost immediately after its discovery.
Named after Pluto, which was “demoted” in 2006, deprived of its planetary status.
Interest in plutonium was initially driven by its military applications. High density and abnormally high compressibility made it possible to produce compact, powerful and structurally simple atomic charges.
All isotopes of plutonium are radioactive. The “reactor” isotope of plutonium makes it possible to create long-lived maintenance-free (up to a hundred years of operation) energy sources.
The most poisonous substance
The most powerful poison is botulinum toxin. We know it under the name Botox, which is what it is called in cosmetology, where it has found its main application. Botulinum toxin is a chemical produced by the bacteria Clostridium botulinum. In addition to the fact that botulinum toxin is the most toxic substance, it also has the largest molecular weight among proteins. The phenomenal toxicity of the substance is evidenced by the fact that only 0.00002 mg•min/l of botulinum toxin is enough to make the affected area deadly to humans for half a day.
Neptunium - 20.47 g/cm³

It was obtained artificially from uranium through nuclear reactions. It is interesting that it was named not in honor of the ancient Greek deity Neptune, but indirectly - due to its practical invisibility in nature in honor of the planet Neptune, which itself was named in honor of the deity, but for a long time was not amenable to observation by astronomers.
This metal has no independent value, but in the radiochemical industry it is a “step” from uranium to the production of the next important radiomaterial - plutonium.
Neutron stars are super-dense space objects
By searching beyond our Earth, we may find the heaviest matter in space in neutron stars.

These are quite unique space inhabitants, one of the possible types of stellar evolution. The diameter of such objects ranges from 10 to 200 kilometers, with a mass equal to our Sun or 2-3 times more.
This cosmic body mainly consists of a neutron core, which consists of flowing neutrons. Although, according to some scientists’ assumptions, it should be in a solid state, reliable information does not exist today. However, it is known that it is neutron stars, reaching their compression limit, that subsequently turn into supernovae with a colossal release of energy, on the order of 1043-1045 joules.
The density of such a star is comparable, for example, to the weight of Mount Everest placed in a matchbox. This is hundreds of billions of tons in one cubic millimeter. For example, to make it more clear how high the density of matter is, let’s take our planet with its mass of 5.9 × 1024 kg and “turn” it into a neutron star.
As a result, in order for the density of the Earth to be equal to the density of a neutron star, it must be reduced to the size of an ordinary apple, with a diameter of 7-10 centimeters. The density of unique stellar objects increases as you move toward the center.
Rhenium - 21.01 g/cm³

Named after the Rhine River, the site of its discovery.
Very rare, the only economically viable rhenium deposit is located in Russia.
Refractoriness, chemical neutrality and good ductility allow this metal to be used to create medical instruments.
Heat-resistant alloys of rhenium with other metals are used in the production of jet engines. Thus, rhenium is of great military and strategic importance.
Platinum - 21.40 g/cm³

The name platinum was invented by the conquistadors. Literally from Spanish it means “silver”. This disparaging name is explained by the special refractoriness of the metal. For many years they did not know how to use it; then platinum cost half as much as silver.
Nowadays it is valued much more than even gold. Extreme refractoriness, chemical inertness and excellent properties as a catalyst for chemical reactions make it indispensable in industry. At the same time, high cost and good strength open up ways for use in jewelry.
Black holes in the Universe
You should pay attention to what is already open today. These are black holes. Perhaps these mysterious objects may be candidates for the fact that the heaviest matter in the Universe is their component. Note that the gravity of black holes is so strong that light cannot escape.

According to scientists, matter drawn into the space-time region becomes so dense that there is no space left between elementary particles.
Unfortunately, beyond the event horizon (the so-called boundary where light and any object, under the influence of gravity, cannot leave a black hole), our guesses and indirect assumptions based on the emission of particle streams follow.
A number of scientists suggest that space and time mix beyond the event horizon. There is an opinion that they may be a “passage” to another Universe. Perhaps this is true, although it is quite possible that beyond these limits another space opens up with completely new laws. An area where time exchanges “place” with space. The location of the future and the past is determined simply by the choice of following. Like our choice to go right or left.
It is potentially possible that there are civilizations in the Universe that have mastered time travel through black holes. Perhaps in the future people from planet Earth will discover the secret of traveling through time.