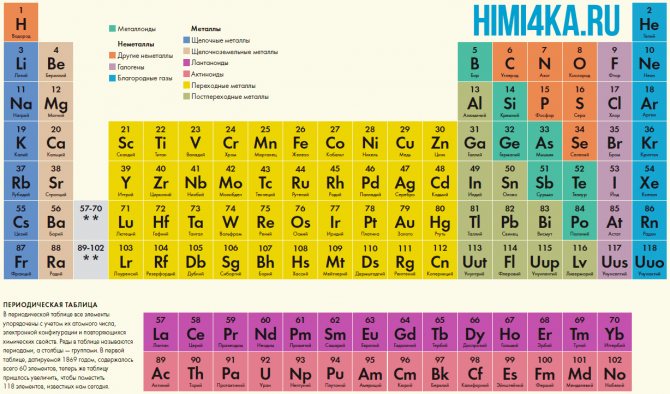
Even at school, sitting in chemistry lessons, we all remember the table on the wall of the classroom or chemical laboratory. This table contained a classification of all chemical elements known to mankind, those fundamental components that make up the Earth and the entire Universe. Then we could not even imagine that the periodic table is undoubtedly one of the greatest scientific discoveries, which is the foundation of our modern knowledge of chemistry.
Periodic table of chemical elements by D. I. Mendeleev
At first glance, her idea looks deceptively simple: organize chemical elements in order of increasing weight of their atoms. Moreover, in most cases it turns out that the chemical and physical properties of each element are similar to the element preceding it in the table. This pattern appears for all elements except the very first few, simply because they do not have in front of them elements similar to them in atomic weight. It is thanks to the discovery of this property that we can place a linear sequence of elements in a table much like a wall calendar, and thus combine a huge number of types of chemical elements in a clear and coherent form. Of course, today we use the concept of atomic number (the number of protons) in order to order the system of elements. This helped solve the so-called technical problem of a “pair of permutations”, but did not lead to a fundamental change in the appearance of the periodic table.
In the periodic table, all elements are ordered based on their atomic number, electronic configuration, and repeating chemical properties. The rows in the table are called periods, and the columns are called groups. The first table, dating back to 1869, contained only 60 elements, but now the table had to be enlarged to accommodate the 118 elements we know today.
Mendeleev's periodic system systematizes not only the elements, but also their most diverse properties. It is often enough for a chemist to have the Periodic Table in front of his eyes in order to correctly answer many questions (not only exam questions, but also scientific ones).
The YouTube ID of 1M7iKKVnPJE is invalid.
Periodic law
There are two formulations of the periodic law of chemical elements: classical and modern.
Classical, as presented by its discoverer D.I. Mendeleev: the properties of simple bodies, as well as the forms and properties of compounds of elements, are periodically dependent on the values of the atomic weights of the elements .
Modern: the properties of simple substances, as well as the properties and forms of compounds of elements, are periodically dependent on the charge of the nucleus of the atoms of the elements (ordinal number) .
A graphic representation of the periodic law is the periodic system of elements, which is a natural classification of chemical elements based on regular changes in the properties of elements depending on the charges of their atoms. The most common images of the periodic table of elements are D.I. Mendeleev's forms are short and long.

Nonmetals (reactive)
Under normal conditions, boron, carbon, phosphorus, sulfur, and selenium are solids of various colors. Each of them has different forms of crystal lattice (allotropic modifications), for example red, yellow, black, white phosphorus, which greatly affects their chemical activity (for example, graphite is much less active than ordinary coal) and physical properties (diamond is the hardest substance in nature and is a transparent material, while graphite is brittle, soft, opaque).
Nitrogen and oxygen are gases. At the same time, nitrogen is quite chemically inert, oxygen, on the contrary, is a very active oxidizing agent.
Halides have different physical properties (fluorine, chlorine are gases, bromine is a liquid, iodine is a solid). Astatine is obtained only in extremely small quantities, like tennessine, their properties are poorly studied. Chemically, halides are very strong oxidizing agents (fluorine is the strongest on the periodic table). With metals they usually form salts with an ionic lattice, and with hydrogen they form very strong acids. With nonmetals they also form many polyatomic compounds, usually with covalent bonds.
Groups and periods of the Periodic Table
Groups are vertical rows in the periodic table. In groups, elements are combined based on the highest oxidation state in their oxides. Each group consists of a main and secondary subgroup. The main subgroups include elements of small periods and elements of large periods with the same properties. Side subgroups consist only of elements of large periods. The chemical properties of the elements of the main and secondary subgroups differ significantly.
A period is a horizontal row of elements arranged in increasing order of atomic (atomic) numbers. There are seven periods in the periodic system: the first, second and third periods are called small, they contain 2, 8 and 8 elements, respectively; the remaining periods are called large: in the fourth and fifth periods there are 18 elements, in the sixth - 32, and in the seventh (not yet completed) - 31 elements. Each period, except the first, begins with an alkali metal and ends with a noble gas.
The physical meaning of the serial number of a chemical element: the number of protons in the atomic nucleus and the number of electrons rotating around the atomic nucleus are equal to the serial number of the element.
Non-metals (inert gases)
Extremely chemically passive substances. The first xenon compound, XePtF6, was synthesized in 1962, but neon and helium compounds are still unknown. Nevertheless, inert gases play a huge role in various industries, medicine, diving, etc.
PS: It’s interesting that the name “Mendeleev’s Table” is known mainly only in Russia, while in the rest of the world it is most often called simply “Periodic Table”.
PPS: The article will be supplemented and corrected.
Properties of the periodic table
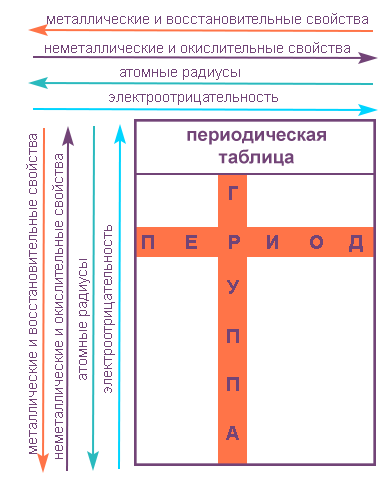
Let us recall that groups are vertical rows in the periodic table and the chemical properties of the elements of the main and secondary subgroups differ significantly.
The properties of elements in subgroups naturally change from top to bottom:
- metallic properties increase and non-metallic properties weaken;
- the atomic radius increases;
- the strength of bases and oxygen-free acids formed by the element increases;
- electronegativity decreases.
All elements except helium, neon and argon form oxygen compounds; there are only eight forms of oxygen compounds. In the periodic table, they are often represented by general formulas, located under each group in order of increasing oxidation state of the elements: R2O, RO, R2O3, RO2, R2O5, RO3, R2O7, RO4, where the symbol R denotes an element of this group. The formulas of higher oxides apply to all elements of the group, except in exceptional cases when the elements do not exhibit an oxidation state equal to the group number (for example, fluorine).
Oxides of the composition R2O exhibit strong basic properties, and their basicity increases with increasing atomic number; oxides of the composition RO (with the exception of BeO) exhibit basic properties. Oxides of the composition RO2, R2O5, RO3, R2O7 exhibit acidic properties, and their acidity increases with increasing atomic number.
The elements of the main subgroups, starting from group IV, form gaseous hydrogen compounds. There are four forms of such compounds. They are located under the elements of the main subgroups and are represented by general formulas in the sequence RH4, RH3, RH2, RH.
RH4 compounds are neutral in nature; RH3 - weakly basic; RH2 - slightly acidic; RH - strongly acidic in nature.
Let us recall that a period is a horizontal series of elements arranged in increasing order of atomic numbers.
Within a period with increasing element serial number:
- electronegativity increases;
- metallic properties decrease, non-metallic properties increase;
- the atomic radius decreases.
Elements of the periodic table
Alkali and alkaline earth elements
These include elements from the first and second groups of the periodic table. Alkali metals from the first group are soft metals, silver in color, easy to cut with a knife. They all have a single electron in their outer shell and react perfectly. Alkaline earth metals from the second group also have a silvery tint. Two electrons are placed at the outer level, and, accordingly, these metals interact less readily with other elements. Compared to alkali metals, alkaline earth metals melt and boil at higher temperatures.
Show/Hide text
| Alkali metals | Alkaline earth metals |
| Lithium Li 3 | Beryllium Be 4 |
| Sodium Na 11 | Magnesium Mg 12 |
| Potassium K 19 | Calcium Ca 20 |
| Rubidium Rb 37 | Strontium Sr 38 |
| Cesium Cs 55 | Barium Ba 56 |
| France Fr 87 | Radium Ra 88 |
Lanthanides (rare earth elements) and actinides
Lanthanides are a group of elements originally found in rare minerals; hence their name "rare earth" elements. Subsequently, it turned out that these elements are not as rare as initially thought, and therefore the name lanthanides was given to rare earth elements. Lanthanides and actinides occupy two blocks, which are located under the main table of elements. Both groups include metals; all lanthanides (except promethium) are non-radioactive; actinides, on the contrary, are radioactive.
Show/Hide text
| Lanthanides | Actinides |
| Lantan La 57 | Actinium Ac 89 |
| Cerium Ce 58 | Thorium Th 90 |
| Praseodymium Pr 59 | Protactinium Pa 91 |
| Neodymium Nd 60 | Uranium U 92 |
| Promethium Pm 61 | Neptunium Np 93 |
| Samaria Sm 62 | Plutonium Pu 94 |
| Europium Eu 63 | Americium Am 95 |
| Gadolinium Gd 64 | Curium Cm 96 |
| Terbium Tb 65 | Berkeley Bk 97 |
| Dysprosium Dy 66 | California Cf 98 |
| Holmium Ho 67 | Einsteinium Es 99 |
| Erbium Er 68 | Fermium Fm 100 |
| Thulium Tm 69 | Mendelevium Md 101 |
| Ytterbium Yb 70 | Nobelium No. 102 |
Halogens and noble gases
The halogens and noble gases are grouped into groups 17 and 18 of the periodic table. Halogens are non-metallic elements and all have seven electrons in their outer shell. In noble gases, all the electrons are in the outer shell, so they hardly participate in the formation of compounds. These gases are called “noble” gases because they rarely react with other elements; that is, they refer to members of the noble caste who have traditionally shunned other people in society.
Show/Hide text
| Halogens | Noble gases |
| Fluorine F 9 | Helium He 2 |
| Chlorine Cl 17 | Neon Ne 10 |
| Bromine Br 35 | Argon Ar 18 |
| Iodine I 53 | Krypton Kr 36 |
| Astatine At 85 | Xenon Xe 54 |
| — | Radon Rn 86 |
Transition metals
Transition metals occupy groups 3–12 on the periodic table. Most of them are dense, hard, with good electrical and thermal conductivity. Their valence electrons (with the help of which they are connected to other elements) are located in several electron shells.
Show/Hide text
| Transition metals |
| Scandium Sc 21 |
| Titan Ti 22 |
| Vanadium V 23 |
| Chrome Cr 24 |
| Manganese Mn 25 |
| Iron Fe 26 |
| Cobalt Co 27 |
| Nickel Ni 28 |
| Copper Cu 29 |
| Zinc Zn 30 |
| Yttrium Y 39 |
| Zirconium Zr 40 |
| Niobium Nb 41 |
| Molybdenum Mo 42 |
| Technetium Tc 43 |
| Ruthenium Ru 44 |
| Rhodium Rh 45 |
| Palladium Pd 46 |
| Silver Ag 47 |
| Cadmium Cd 48 |
| Lutetium Lu 71 |
| Hafnium Hf 72 |
| Tantalum Ta 73 |
| Tungsten W 74 |
| Rhenium Re 75 |
| Osmium Os 76 |
| Iridium Ir 77 |
| Platinum Pt 78 |
| Gold Au 79 |
| Mercury Hg 80 |
| Lawrence Lr 103 |
| Rutherfordium Rf 104 |
| Dubnium Db 105 |
| Seaborgium Sg 106 |
| Borium Bh 107 |
| Hassiy Hs 108 |
| Meitnerium Mt 109 |
| Darmstadt Ds 110 |
| X-ray Rg 111 |
| Copernicium Cn 112 |
Metalloids
Metalloids occupy groups 13-16 of the periodic table. Metalloids such as boron, germanium and silicon are semiconductors and are used to make computer chips and circuit boards.
Show/Hide text
| Metalloids |
| Boron B 5 |
| Silicon Si 14 |
| Germanium Ge 32 |
| Arsenic As 33 |
| Antimony Sb 51 |
| Tellurium Te 52 |
| Polonium Po 84 |
Post-transition metals
The elements, called post-transition metals , belong to groups 13–15 of the periodic table. Unlike metals, they do not have shine, but have a matte color. Compared to transition metals, post-transition metals are softer, have lower melting and boiling points, and higher electronegativity. Their valence electrons, with which they attach other elements, are located only on the outer electron shell. Post-transition metal group elements have much higher boiling points than metalloids.
Show/Hide text
| Post-transition metals |
| Aluminum Al 13 |
| Gallium Ga 31 |
| Indium In 49 |
| Tin Sn 50 |
| Thallium Tl 81 |
| Lead Pb 82 |
| Bismuth Bi 83 |
Physical parameters of the metal
From a physics point of view, gold also has many advantages over other metals. Pure gold has a bright yellow rich color. If the product has a tint, ask what is included in the ligature. The addition of copper gives a reddish tint, silver or other white metal in the alloy creates white gold. In nature, in a gold deposit, you can see a precious metal with a green tint, depending on the amount of ore included in the composition.
In terms of hardness, gold is considered a soft metal. The scale with criteria is named after Mohs, and the hardest substance on earth is considered to be diamond. It was assigned the number 10. For gold, this figure is 2.5-3.0. This is because pure gold can easily be cut with a knife or even just scratched. It is because of this that jewelers do not want to make jewelry from 999 fineness, since it is short-lived and the product will need to be additionally covered with protective layers on top.
To determine the hardness of a metal in the field, it is enough to scratch it with a sharp object. If a trace remains, then the hardness is below 5. This is also how the authenticity of coins was checked in the old days. They were simply bitten, and if traces remained on the money, this means that the coins were real.
Gold can splinter and scatter, as well as wear off. Therefore, coins made of pure precious metal quickly failed and became unsuitable for trade. The metal is easy to polish and can be used as a light reflector.
The metal has high malleability, ductility and ductility. It is thanks to these properties that jewelry and products of various shapes are made from gold. It is even possible to roll out thin sheets of metal. This type of gold is called leaf and is used to cover icons and interior items.
Metal is used to create very small chips in microcircuits. In addition, in addition to being malleable, gold conducts electricity well, and the contacts do not move away from each other over time. Gold can be melted at a temperature of 1063 degrees Celsius, and boiled at 2947 degrees Celsius. During these processes, the metal will lose its color and turn pale green.
The property of the metal, its density, helps gold miners. It is 19.3 times greater for gold than for water. Therefore, gold particles settle at the bottom of running water, and they are also easy to rinse and remove impurities.