| Rhodium | |
| Atomic number | 45 |
| Appearance of a simple substance | silver-white hard metal |
| Properties of the atom | |
| Atomic mass (molar mass) | 102.9055 a. e.m. (/mol) |
| Atomic radius | 134 |
| Ionization energy (first electron) | 719.5 (7.46) kJ/mol () |
| Electronic configuration | [Kr] 4d8 5s1 |
| Chemical properties | |
| Covalent radius | 125 pm |
| Ion radius | (+3e)68 pm |
| Electronegativity (Pauling) | 2,28 |
| Electrode potential | 0 |
| Oxidation states | 5, 4, 3, 2, 1, 0 |
| Thermodynamic properties of a simple substance | |
| Density | 12.41 g/cm³ |
| Molar heat capacity | 24.95 J/(K mol) |
| Thermal conductivity | 150 W/(m K) |
| Melting temperature | 2239 K |
| Heat of Melting | 21.8 kJ/mol |
| Boiling temperature | 4000 K |
| Heat of vaporization | 494 kJ/mol |
| Molar volume | 8.3 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | a=3.803 Å |
| c/a ratio | — |
| Debye temperature | 480K |
| Rh | 45 |
| 102,9055 | |
| [Kr]4d85s1 | |
| Rhodium | |
Rhodium
- an element of the side subgroup of the eighth group of the fifth period of the periodic system of chemical elements, atomic number 45. Denoted by the symbol Rh (Latin Rhodium). The element rhodium (CAS number: 7440-16-6) is a hard, silvery-white transition metal. A noble metal of the platinum group.
Physical properties
Rhodium foil and wire
Rhodium is a hard metal, silver-gray in color. It has a high reflectivity of electromagnetic rays of the visible part of the spectrum, therefore it is widely used for the manufacture of “surface” mirrors.
Isotopes of rhodium
Natural rhodium consists of the isotope 103Rh. Longest-lived isotopes
| Isotope | Half life |
| 101Rh | 3.3 years |
| 102Rh | 207 days |
| 102mRh | 2.9 years |
| 99Rh | 16.1 days |
Content
- 1. History
- 2 Origin of the name
- 3 Occurrence in nature 3.1 Deposits
- 8.1 Catalysts
Chemical properties
Rhodium is a noble metal; its chemical resistance in most corrosive environments is superior to platinum. Metallic rhodium dissolves in aqua regia when boiled, as well as electrochemically, anodically - in a mixture of hydrogen peroxide and sulfuric acid.
Rhodium is characterized by high chemical resistance. It interacts with non-metals only at red heat temperatures. Finely ground rhodium slowly oxidizes only at temperatures above 600°C:
4Rh + 3O2 = 2Rh2O3.
When heated, rhodium slowly reacts with concentrated sulfuric acid, a solution of sodium hypochlorite and hydrogen bromide. During sintering, it reacts with molten potassium hydrogen sulfate KHSO4, sodium peroxide Na2O2 and barium peroxide BaO2:
2Rh + 6KHSO4 = 2K3Rh(SO4)3 + 3H2↑,
2Rh + 3BaO2 = Rh2O3 + 3BaO.
In the presence of alkali metal chlorides, when it is possible to form [RhX3]3- complexes, rhodium reacts with chlorine, for example:
2Rh + 6NaCl + Cl2 = 2Na3[RhCl6].
When aqueous solutions of rhodium(III) salts and complexes are exposed to alkalis, a precipitate of rhodium hydroxide Rh(OH)3 is formed:
Na3[RhCl6] + 3NaOH = Rh(OH)3↓ + 6NaCl.
Rhodium(III) hydroxide and oxide exhibit basic properties and react with acids to form Rh(III) complexes:
Rh2O3 + 12HCl = 2H3RhCl6 + 3H2O,
Rh(OH)3 + 6HCl = H3RhCl6 + 3H2O.
Rhodium exhibits the highest oxidation state +6 in the hexafluoride RhF6, which is formed by direct combustion of rhodium in fluorine. The connection is unstable. In the absence of water vapor, hexafluoride oxidizes free chlorine or nitrogen oxide (II) NO:
2RhF6 + 3Cl2 = 2RhF3 + 6ClF.
In the lower oxidation states +1 and +2, rhodium forms complex compounds.
Receipt
Rhodium is extracted from native platinum[4]. Raw native platinum is placed in porcelain cauldrons, after which it is treated with aqua regia while heating for 24 hours. Rhodium, almost all platinum, palladium, base metals (iron, copper and others), partially ruthenium and iridium go into solution, and osmic iridium, quartz, chromium iron ore and other impurities remain in the sediment. The remaining solution is evaporated, up to 6% rhodium remains in the sediment; palladium, ruthenium, iridium, platinum are also present (all of it cannot be separated using NH4Cl) and base metals. This precipitate is dissolved in water and the platinum is separated again in the same way. The solution, in which rhodium, ruthenium and palladium remain, is sent for purification and separation.
Rhodium is extracted in different ways. There is a known method proposed by the Soviet scientist V.V. Lebedinsky in 1932. First, the solution is treated with sodium nitrite NaNO2. In this way, base metal hydroxides are precipitated and separated from the solution. Rhodium is retained in solution in the form of Na3[Rh(NO2)6]. After this, the action of NH4Cl on the solution in the cold releases rhodium in the form of a poorly soluble complex (NH4)2Na[Rh(NO2)6]. However, in this case, along with rhodium, iridium also precipitates. The other platinum metals—ruthenium, palladium, and residual platinum—remain in solution.
The precipitate is treated with diluted sodium hydroxide, which allows it to dissolve. Rhodium is again precipitated from the resulting solution by the action of ammonia and NH4Cl. Precipitation occurs due to the formation of a poorly soluble complex compound [Rh(NH3)3(NO2)3]. The separated precipitate is thoroughly washed with ammonium chloride solution. After this, the precipitate is treated with hydrochloric acid, heating it in it for several hours. The reaction occurs:
2[Rh(NH3)3(NO2)3] + 6HCl → 2[Rh(NH3)3Cl3] +3NO2 + 3NO + 3H2O
with the formation of bright yellow rhodium triamine trichloride. The precipitate is thoroughly washed with water, transforming it into a state suitable for the isolation of metallic rhodium. The resulting compound is calcined for several hours at 800–900 °C. The result of the process is a powdery product of a mixture of rhodium and its oxides. The powder is cooled, washed with diluted aqua regia to remove the remaining small amount of base impurities, and then at high temperature it is reduced to metal in a hydrogen environment.
Application
Catalysts
- Rhodium is used in catalysts, including catalytic filter-neutralizers for automobile exhaust gases
- The rhodium-platinum alloy is a very effective catalyst for the production of nitric acid by the oxidation of ammonia with air, and there is still no alternative replacement for its use.
Construction material
- in glass production (platinum-rhodium alloy - dies for glass filaments, for liquid crystal screens). Due to the growth in the production of liquid crystal devices, the consumption of rhodium is growing rapidly (in 2005, 1.55 tons of rhodium were used in glass production, in 2003 - 0.81 tons).
- Metallic rhodium is used for the production of mirrors subjected to strong heating (incandescence) for high-power laser systems (for example, hydrogen fluoride lasers), as well as for the production of diffraction gratings for devices for analyzing substances (spectrometers).
- Platinum-rhodium alloy crucibles are used in laboratory research and for growing some gemstones and electro-optical crystals.
Thermocouple
- Platinum-rhodium thermocouples, etc., rhodium-iridium alloys (for example IR 40\60) are widely used as very effective and durable measurements of high (up to 2200 °C) temperatures.
Contact pair material
Due to its high resistance to electrical erosion, rhodium and its alloys are used as a material for contacts (reed switches, connectors, sliding contacts).
Jewelry
Galvanic rhodium plating electrolytes (mainly sulfate, sulfamate and phosphate) are used to obtain wear-resistant and corrosion-resistant coatings.
The cool white shine of rhodium goes well with diamonds, cubic zirconia and other inserts. Rhodium is also added as an alloying and strengthening additive to platinum and palladium. Applying rhodium plating to jewelry reduces wear and increases the hardness of the piece, protects it from scratches, and adds a bright shine.
Content in nature
Rhodium is a very rare and trace element. Only the 103Rh isotope occurs in nature. The average content of rhodium in the earth's crust is 1·10−7% by mass, in stone meteorites 4.8·10−5%. Rhodium content is elevated in ultramafic igneous rocks. It does not have its own minerals. Found in some of the golden sands of South America. Contained in nickel and platinum ores in the form of a simple compound. Up to 43% of rhodium comes from Mexican gold deposits. It is also found in isomorphic admixtures of minerals of the iridium osmide group (up to 3.3%) and in copper-nickel ores. A rare variety of osmic iridium - rhodium nevyanskite - is the richest mineral in rhodium (up to 11.3%).
Place of Birth
Less than 30 tons of rhodium are mined annually in the world. Rhodium deposits are located in South Africa, Canada, Colombia, and Russia[4].
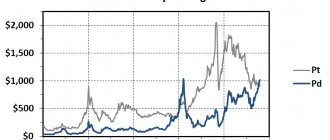
Prices
In February 2006, rhodium prices reached a record high of $3,500 per troy ounce according to kitco.com/charts/rhodium.html. In January 2008, rhodium prices set a new record of $7,000 per ounce. After peaking at $10,100 per ounce, the price of rhodium fell to $900 at the end of November 2008 due to the crisis in the automotive industry. On November 19, 2009, the price of the metal rose to $2,600 per ounce. Due to very high prices for pure rhodium and with significant demand and a small volume of mined rhodium, an urgent task arises to solve the acute shortage of rhodium, isolating its stable isotope from fission fragments of nuclear fuel (uranium, plutonium, thorium), where it accumulates in significant quantities. quantities (up to 130-180 grams per ton of fragments), and taking into account the developed nuclear energy in the largest industrial countries, the volume of production of reactor rhodium will be several times higher than its production from ores. In addition, research is also needed on the issue of reactor operating modes, in which the amount of rhodium as a percentage of the mass of fragments will be higher, and thus the nuclear industry can become the main supplier of rhodium to the world market.
Rhodium plating technology
Due to its special, pleasant silver-white hue and amazing reflectivity, the element is very much in demand by jewelers. Rhodium plating allows not only to impart a brilliant platinum shimmer, but also to increase the strength of products and their resistance to scratches. The application process is a lengthy procedure that requires preliminary preparation.
Before coating, the jewelry is polished until there is no evidence left on the metal surface that it has already been worn. The next stage is degreasing and disinfection. After this, it is sent to a galvanic bath, where a rhodium layer is applied.
A solution containing positively charged particles of metal salts is first poured into a special container. In this case, the object itself is charged with negative particles. An electric charge is supplied through it, as a result of which opposite particles are attracted to each other. The jewelry itself acts as an anode and, at the moment of current action, is completely covered and disappears under the rhodium. The longer and more intense the charge is applied, the thicker the coating becomes.
Several years ago, the main element used in this technology was rhodium salts. However, due to the difficulty of their extraction, in our time they have been replaced by rhodium sulfate, which is produced specifically for creating a coating on products.
Despite its fairly high strength, the coating layer applied to jewelry can be damaged during cleaning using abrasives. Therefore, it is better to take them off to maintain a flawless appearance. To care for such items, you can use soft wet wipes . It should be remembered that the rhodium plating procedure is a temporary measure, so there may be a need for repeated processing.
Excerpt characterizing Rhodium
The next day, Davout left early in the morning and, inviting Balashev to his place, impressively told him that he asked him to stay here, move along with the luggage if they had orders to do so, and not talk to anyone except Mister de Castro. After four days of solitude, boredom, a sense of subordination and insignificance, especially palpable after the environment of power in which he had so recently found himself, after several marches along with the marshal’s luggage, with the French troops occupying the entire area, Balashev was brought to Vilna, now occupied by the French , to the same outpost where he left four days ago. The next day, the imperial chamberlain, monsieur de Turenne, came to Balashev and conveyed to him the desire of Emperor Napoleon to honor him with an audience. Four days ago, at the house to which Balashev was taken, there were sentries of the Preobrazhensky Regiment, but now there were two French grenadiers in blue uniforms open on their chests and in shaggy hats, a convoy of hussars and lancers and a brilliant retinue of adjutants, pages and generals waiting to leave Napoleon around a riding horse standing at the porch and his Mameluke Rustav. Napoleon received Balashev in the very house in Vilva from which Alexander sent him. Despite Balashev's habit of court solemnity, the luxury and pomp of Emperor Napoleon's court amazed him. Count Turen led him into a large reception room, where many generals, chamberlains and Polish magnates were waiting, many of whom Balashev had seen at the court of the Russian emperor. Duroc said that Emperor Napoleon would receive the Russian general before his walk. After several minutes of waiting, the chamberlain on duty came out into the large reception room and, bowing politely to Balashev, invited him to follow him. Balashev entered a small reception room, from which there was one door to an office, the very office from which the Russian emperor sent him. Balashev stood there for about two minutes, waiting. Hasty steps were heard outside the door. Both halves of the door quickly opened, the chamberlain who opened it stopped respectfully, waiting, everything became quiet, and other, firm, decisive steps sounded from the office: it was Napoleon. He had just finished his riding toilet. He was wearing a blue uniform, open over a white vest that hung down over his round belly, white leggings that hugged the fat thighs of his short legs, and boots. His short hair had obviously just been combed, but one strand of hair hung down over the middle of his wide forehead. His white, plump neck protruded sharply from behind the black collar of his uniform; he smelled of cologne. On his youthful, plump face with a prominent chin there was an expression of gracious and majestic imperial greeting. He walked out, shaking quickly with every step and throwing his head back a little. His entire plump, short figure with wide, thick shoulders and an involuntarily protruding belly and chest had that representative, dignified appearance that forty-year-old people living in the hallway have. In addition, it was clear that he was in the best spirits that day. He nodded his head, responding to Balashev’s low and respectful bow, and, approaching him, immediately began to speak like a man who treasures every minute of his time and does not deign to prepare his speeches, but is confident in what he will always say ok and what needs to be said. - Hello, general! - he said. “I received the letter from Emperor Alexander that you delivered, and I am very glad to see you.” “He looked into Balashev’s face with his big eyes and immediately began to look ahead past him. It was obvious that he was not at all interested in Balashev’s personality. It was clear that only what was happening in his soul was of interest to him. Everything that was outside of him did not matter to him, because everything in the world, as it seemed to him, depended only on his will. “I do not want and did not want war,” he said, “but I was forced into it.” Even now (he said this word with emphasis) I am ready to accept all the explanations that you can give me. - And he clearly and briefly began to state the reasons for his displeasure against the Russian government. Judging by the moderately calm and friendly tone with which the French emperor spoke, Balashev was firmly convinced that he wanted peace and intended to enter into negotiations. - Sire! L'Empereur, mon maitre, [Your Majesty! Emperor, my lord,] - Balashev began a long-prepared speech when Napoleon, having finished his speech, looked questioningly at the Russian ambassador; but the look of the emperor's eyes fixed on him confused him. “You are embarrassed - come to your senses,” Napoleon seemed to say, looking at Balashev’s uniform and sword with a barely noticeable smile. Balashev recovered and began to speak. He said that Emperor Alexander did not consider Kurakin’s demand for passports to be a sufficient reason for war, that Kurakin did so arbitrarily and without the consent of the sovereign, that Emperor Alexander did not want war and that there were no relations with England. “Not yet,” Napoleon interjected and, as if afraid to give in to his feelings, he frowned and nodded his head slightly, thereby letting Balashev feel that he could continue. Having expressed everything that was ordered to him, Balashev said that Emperor Alexander wants peace, but will not begin negotiations except on the condition that... Here Balashev hesitated: he remembered those words that Emperor Alexander did not write in the letter, but which he certainly ordered that Saltykov be inserted into the rescript and which Balashev ordered to hand over to Napoleon. Balashev remembered these words: “until not a single armed enemy remains on Russian land,” but some complex feeling held him back. He could not say these words, although he wanted to do so. He hesitated and said: on the condition that the French troops retreat beyond the Neman. Napoleon noticed Balashev's embarrassment when uttering his last words; his face trembled, his left calf began to tremble rhythmically. Without leaving his place, he began to speak in a voice higher and more hasty than before. During the subsequent speech, Balashev, more than once lowering his eyes, involuntarily observed the trembling of the calf in Napoleon’s left leg, which intensified the more he raised his voice. “I wish peace no less than Emperor Alexander,” he began. “Isn’t it me who has been doing everything for eighteen months to get it?” I've been waiting eighteen months for an explanation. But in order to start negotiations, what is required of me? - he said, frowning and making an energetic questioning gesture with his small, white and plump hand. “The retreat of the troops beyond the Neman, sir,” said Balashev. - For Neman? - Napoleon repeated. - So now you want them to retreat beyond the Neman - only beyond the Neman? – Napoleon repeated, looking directly at Balashev. Balashev bowed his head respectfully. Instead of the demand four months ago to retreat from Numberania, now they demanded to retreat only beyond the Neman. Napoleon quickly turned and began to walk around the room. – You say that they require me to retreat beyond the Neman to begin negotiations; but they demanded of me in exactly the same way two months ago to retreat beyond the Oder and Vistula, and, despite this, you agree to negotiate. He silently walked from one corner of the room to the other and again stopped opposite Balashev. His face seemed to harden in its stern expression, and his left leg trembled even faster than before. Napoleon knew this trembling of his left calf. “La vibration de mon mollet gauche est un grand signe chez moi,” he said later. “Such proposals as clearing the Oder and the Vistula can be made to the Prince of Baden, and not to me,” Napoleon almost cried out, completely unexpectedly for himself. – If you had given me St. Petersburg and Moscow, I would not have accepted these conditions. Are you saying I started the war? Who came to the army first? - Emperor Alexander, not me. And you offer me negotiations when I have spent millions, while you are in an alliance with England and when your position is bad - you offer me negotiations! What is the purpose of your alliance with England? What did she give you? - he said hastily, obviously already directing his speech not in order to express the benefits of concluding peace and discussing its possibility, but only in order to prove both his rightness and his strength, and to prove Alexander’s wrongness and mistakes. The introduction of his speech was made, obviously, with the aim of showing the advantage of his position and showing that, despite the fact, he accepted the opening of negotiations. But he had already begun to speak, and the more he spoke, the less able he was to control his speech. The whole purpose of his speech now, obviously, was only to exalt himself and insult Alexander, that is, to do exactly what he least wanted at the beginning of the date. - They say you made peace with the Turks? Balashev tilted his head affirmatively. “The world is concluded...” he began. But Napoleon did not let him speak. He apparently needed to speak on his own, alone, and he continued to speak with that eloquence and intemperance of irritation to which spoiled people are so prone. – Yes, I know, you made peace with the Turks without receiving Moldavia and Wallachia. And I would give these provinces to your sovereign just as I gave him Finland. Yes,” he continued, “I promised and would have given Moldavia and Wallachia to Emperor Alexander, but now he will not have these beautiful provinces.” He could, however, annex them to his empire, and in one reign he would expand Russia from the Gulf of Bothnia to the mouth of the Danube. “Katherine the Great could not have done more,” said Napoleon, becoming more and more excited, walking around the room and repeating to Balashev almost the same words that he said to Alexander himself in Tilsit. – Tout cela il l'aurait du a mon amitie... Ah! quel beau regne, quel beau regne! - he repeated several times, stopped, took a golden snuffbox out of his pocket and greedily sniffed from it. – Quel beau regne aurait pu etre celui de l'Empereur Alexandre! [He would owe all this to my friendship... Oh, what a wonderful reign, what a wonderful reign! Oh, what a wonderful reign the reign of Emperor Alexander could have been!] He looked at Balashev with regret, and just as Balashev wanted to notice something, he again hastily interrupted him. “What could he want and seek that he would not find in my friendship?..” said Napoleon, shrugging his shoulders in bewilderment. - No, he found it best to surround himself with my enemies, and who? - he continued. - He called to him the Steins, Armfelds, Wintzingerode, Bennigsenov, Stein - a traitor driven out of his fatherland, Armfeld - a libertine and intriguer, Wintzingerode - a fugitive subject of France, Bennigsen somewhat more military than the others, but still incapable, who could not do anything to do in 1807 and which should awaken terrible memories in Emperor Alexander... Let’s suppose, if they were capable, they could be used,” continued Napoleon, barely managing to keep up with the words that constantly arise, showing him his rightness or strength (which in in his concept were one and the same) - but even that is not the case: they are not suitable for either war or peace. Barclay, they say, is more efficient than all of them; but I won’t say that, judging by his first movements. What are they doing? What are all these courtiers doing! Pfuhl proposes, Armfeld argues, Bennigsen considers, and Barclay, called to act, does not know what to decide on, and time passes. One Bagration is a military man. He is stupid, but he has experience, an eye and determination... And what role does your young sovereign play in this ugly crowd. They compromise him and blame him for everything that happens. “Un souverain ne doit etre a l'armee que quand il est general, [The sovereign should be with the army only when he is a commander,” he said, obviously sending these words directly as a challenge to the sovereign’s face. Napoleon knew how much Emperor Alexander wanted to be a commander. – It’s already been a week since the campaign began, and you have failed to defend Vilna. You are cut in two and driven out of the Polish provinces. Your army is grumbling... - On the contrary, Your Majesty, - said Balashev, who barely had time to remember what was said to him, and had difficulty following this fireworks of words, - the troops are burning with desire... - I know everything, - Napoleon interrupted him, - I’m all I know, and I know the number of your battalions as surely as mine. You don’t have two hundred thousand troops, but I have three times that much. “I give you my word of honor,” said Napoleon, forgetting that his word of honor could not have any meaning, “I give you ma parole d’honneur que j’ai cinq cent trente mille hommes de ce cote de la Vistule.” [on my word of honor that I have five hundred and thirty thousand people on this side of the Vistula.] The Turks are no help to you: they are no good and have proven this by making peace with you. The Swedes are destined to be ruled by crazy kings. Their king was mad; they changed him and took another - Bernadotte, who immediately went crazy, because a crazy person only being a Swede can enter into alliances with Russia. - Napoleon grinned viciously and again brought the snuffbox to his nose.
Links
Periodic table of chemical elements by D. I. Mendeleev Rh Uut Uup Uus Uuo Uue Ubn Ubu Ubb Ubt Ubq UBP Ubh Alkali metals Alkaline earth metals Lanthanides Actinoids Superactinoids Transition metals Other metals Semimetals Other non-metals Halogens Noble gases Properties unknown
Notes
- Michael E. Wieser, Norman Holden, Tyler B. Coplen, John K. Böhlke, Michael Berglund, Willi A. Brand, Paul De Bièvre, Manfred Gröning, Robert D. Loss, Juris Meija, Takafumi Hirata, Thomas Prohaska, Ronny Schoenberg, Glenda O'Connor, Thomas Walczyk, Shige Yoneda, Xiang-Kun Zhu.
[iupac.org/publications/pac/85/5/1047/ Atomic weights of the elements 2011 (IUPAC Technical Report)] (English) // Pure and Applied Chemistry. — 2013. — Vol. 85, no. 5. - P. 1047-1078. — DOI:10.1351/PAC-REP-13-03-02. - Editorial team: Zefirov N. S. (chief editor).
Chemical encyclopedia: in 5 volumes - Moscow: Soviet Encyclopedia, 1995. - T. 4. - P. 270. - 639 p. — 20,000 copies. — ISBN 5—85270—039—8. - [www.chem-astu.ru/science/reference/potentials/fi.php?a=Rh Directory of redox potentials. Rhodium]. www.chem-astu.ru. Retrieved September 21, 2021.
- ↑ 12
[nt.ru/ri/ps/pb045.htm Popular library of chemical elements. Rhodium. Books. Science and Technology] - [www.kitco.com/charts/rhodium.html Rhodium Charts]
- [www.cmmarket.ru/ World commodity markets: news, reviews, statistics, prices]. www.cmmarket.ru. Retrieved October 31, 2015.