The unique chemical properties of gold have given it a special place among the metals used on Earth. Gold has been known to mankind since ancient times. Since ancient times it has been used as jewelry; alchemists tried to extract the precious metal from other less noble substances. Currently, the demand for it is only growing. It is used in industry, medicine, and technology. In addition, it is acquired by both states and private individuals, using it as an investment metal.
Chemical properties of the “king of metals”
The symbol Au is used to denote gold. This is an abbreviation for the Latin name for the metal - Aurum. In the periodic table of Mendeleev it is number 79 and is located in group 11. In appearance it is a yellow metal. Gold is in the same group with copper, silver and roentgenium, but its chemical properties are closer to the platinum group metals.
Inertness is a key property of this chemical element, which is possible due to the high value of the electrode potential. Under standard conditions, gold does not react with anything except mercury. With it, this chemical element forms an amalgam, which easily disintegrates when heated to just 750 degrees Celsius.
The chemical properties of the element are such that other compounds with it are also short-lived. This property is actively used in the extraction of precious metals. The reactivity of gold increases significantly only with intense heating. For example, it can be dissolved in chlorine or bromine water, an alcohol solution of iodine and, of course, in aqua regia - a mixture of hydrochloric and nitric acid in a certain proportion. The chemical formula for the reaction of such a compound is: 4HCl + HNO3 + Au = H (AuCl4) + NO + 2H2.
The chemistry of gold is such that when heated it can react with halogens. To form gold salts, this chemical element must be reduced from an acidic solution. In this case, the salts will not precipitate, but will dissolve into liquid, forming colloidal solutions of different colors.
Despite the fact that gold does not enter into active chemical reactions with substances, in everyday life you should not allow products made from it to interact with mercury, chlorine and iodine. Various household chemicals are also not the best companion for precious metal products.
The fact is that jewelry uses an alloy of gold with other metals, and various substances, interacting with these impurities, can cause irreparable damage to the beauty of the product. If you heat gold above 100 degrees Celsius, an oxide film one millionth of a millimeter thick will appear on its surface.
Counterfeiters
The general passion for alchemy has given rise to a great many deceivers. Various methods were used to deceive. For example, to demonstrate the transformation of lead into gold, a gold bar was taken that was previously coated with lead. When heated, the lead melted and revealed pure gold! Sometimes crucibles with double bottoms were installed. Or they hid pieces of gold in wooden sticks, which were used to stir molten lead or mercury. The gold that appeared in the melts proved the fact of the transformation. An alchemist caught cheating was usually hanged as a counterfeiter - on a gilded gallows and in a robe strewn with sequins. If the alchemist was not caught in deception, it was believed that he really possessed the secret - the philosopher's stone - and then a dungeon awaited him. It was a vicious circle from which it was impossible to get out alive. Therefore, practicing alchemy required considerable courage.
Other features of the precious metal
Gold is one of the heaviest metals known. Its density is 19.3 g/cm3. An ingot weighing 1 kilogram has very small dimensions, 8x4x1.8 centimeters. This is the standard size of a bank gold bar of this weight. It is comparable to the size of a regular credit card, although the bar is slightly thicker.
Only a few elements are heavier than gold: plutonium, osmium, iridium, platinum and rhenium. But their content in the earth’s crust, even taken together, is much less than this precious metal. Moreover, plutonium (chemical symbol Pu, not to be confused with Pt - this is the symbol of platinum) is a radioactive element.

The chemical composition of gold provides its physical properties. So, the main properties of this metal that make it unique include:
- Malleability, plasticity, ductility. It is very easy to flatten or stretch. So, from just one gram of gold you can get a wire 3 kilometers long, and the area of thin sheets obtained from 1 kilogram will be 530 square meters. Ultra-thin sheets of gold foil are called “gold leaf.” They cover, for example, church domes and the interior decoration of palaces. Thanks to its plasticity, a small amount of yellow metal can cover gigantic areas.
- Softness. High-grade gold is so soft that it can be easily scratched even with a fingernail. This is why bullion in jars is sold in sealed plastic packaging. If even one small scratch is noticed on it, it will be considered defective. In order to make gold more durable, other metals are added to it when making products. This property has ensured the high popularity of the king of metals in the jewelry industry.
- High electrical conductivity. Due to this chemical property, gold is highly valued in electrical engineering and industry. Only silver and copper conduct electricity better than it. At the same time, gold hardly heats up: in terms of thermal conductivity, diamond, silver and copper are higher than it. Together with its property of oxidation resistance, gold is an ideal substance for the manufacture of semiconductors.
- Reflection of infrared light. The finest gold plating applied to the glass does not transmit infrared radiation, leaving the visible part of the spectrum. This property is actively used in astronautics when it is necessary to protect the eyes of astronauts from the harmful effects of the sun. Spraying is often used in the mirror system of high-rise buildings to reduce the cost of cooling rooms.
- Resistant to corrosion and oxidation. Ingots that are stored in accordance with the rules are practically not subject to any chemical influence even when exposed to air. So the greater preservation of gold ensured its high popularity.
Gold mining method
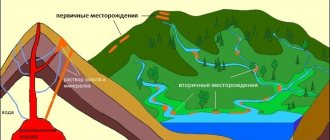
Gold is a fairly rare element on Earth. Its content in the earth's crust is low. It is mainly found in the form of placers in the native state or in the form of ore and occasionally occurs in the form of minerals. Sometimes gold is mined as a by-product in the development of copper or polymetallic ores.
Humanity knows many ways to extract this noble metal. The simplest is elutriation, that is, the separation of gold ore from waste rock using a special technical process. However, this method involves large losses, since the technology is far from perfect. The mechanical method of extracting gold ore was replaced by chemistry. Alchemists, and after them chemists, received many methods for isolating the desired metal from a rock, among them the most common:
- amalgamation;
- cyanidation;
- electrolysis.
Electrolysis, discovered in 1896 by E. Wohlwill, has become widespread in industry. Its essence lies in the fact that anodes consisting of a gold-containing substance are placed in a bath with hydrochloric acid solution. A sheet of pure gold is used as a cathode. During the process of electrolysis (passing current through the cathode and anode), the desired substance is deposited on the cathode, and all impurities precipitate. Thus, the chemical properties of the precious metal help to obtain it on an industrial scale with virtually no losses.
Edison. Tesla and others
The great Edison and the mysterious Nikola Tesla also did not escape the temptation of searching for the philosopher’s stone.” For many days, secluded in their laboratory, they indulged in unknown activities. In order to protect themselves from the idle curiosity of fanatics, the researchers carefully curtained the laboratory windows when they left. Edison personally checked the locks on the doors. Hoping for the expected result, they, in particular, irradiated thin silver plates using an X-ray machine with gold electrodes. But luck came to the professional chemist Stephen Emmens, by the way, the author of the invention of the explosive for mines - “emmensite”. He told the press that he had discovered a way to transform silver into a metal almost indistinguishable from gold! Oddly enough, but three ingots of this metal, after laboratory tests, were bought by goldsmiths at the price of real gold! The last thing that can be said about Stephen Emmens is that he agreed to give a public demonstration of his experiments at the World Exhibition in Paris in 1900. But he never appeared there, and completely disappeared without a trace! A similar fate befell the German professor Adolf Miethe, who in 1924 announced his invention of a method for converting mercury into gold. Using this method, the professor allegedly repeatedly obtained pure gold in his laboratory!
Alloys with other metals
Noble metal alloys are formed for two purposes:
- Change the mechanical properties of gold, make it stronger or, conversely, more brittle and malleable.
- Save precious metal reserves.
Various additions to gold are called alloys. The color and properties of the alloy depend on the chemical formula of its components. Thus, silver and copper significantly increase the hardness of the alloy, which makes it possible to use it for making jewelry. But lead, platinum, cadmium, bismuth and some other chemical elements make the alloy more brittle. Despite this, they are often used to produce the most expensive jewelry, as they significantly change the color of the product. The most common alloys:

- green gold - an alloy of 75% gold, 20% silver and 5% indium;
- white gold is an alloy of gold and platinum (in a ratio of 47:1) or gold, palladium and silver in a ratio of 15:4:1.
- red gold - an alloy of gold (78%) and aluminum (22%);
- an alloy of gold and silver in a ratio of 3:1 (interestingly, an alloy in any other proportion will become white, and these alloys are called by the general term “electron”).
Depending on the amount of gold in the alloy, its sample is determined. It is measured in ppm and is indicated by a three-digit number. The amount of the required metal in each alloy is strictly regulated by the state. In Russia, only 5 samples are officially accepted: 375, 500, 585, 750, 958, 999. The sample numbers mean that this is exactly how many measures of gold there are per 1000 measures of the alloy.
In other words, a 585-carat bar or product contains 58.5% gold. Gold of the highest standard, 999, is considered pure. Only chemistry uses it for its needs, since this metal is too fragile and soft. 750 standard is the most popular in the jewelry industry. Its main components are silver, copper, platinum. The product must have a mark - a digital sign indicating the sample.
GOLD
GOLD (Aurum) Au, chemical. element I gr. periodic systems, at. n. 79, at. m. 196.9665; belongs to the noble metals. There is only one stable isotope in nature, 197Au. External configuration electron shell 5d106s1; oxidation states +1, +3, rarely +5; ionization energies Au0 : Au+ : Au2+ : Au3+ resp. equal to 9.2258, 20.5 and 30.5 eV; Pauling electronegativity 2.4 electron affinity 2.8 eV; atomic radius 0.144 nm, ionic radii (coordination numbers are indicated in parentheses) Au+ 0.151 nm (6), Au3+ 0.082 nm (4), 0.099 nm (6). The gold content in the earth's crust is 4.3-10% by weight, in the water of the seas and oceans it is less than 5.10-6 mg/l. Refers to scattered elements. More than 20 minerals are known, of which the main one is native gold (electrum, cuprous, palladium, bismuth gold, etc.), which is a solid solution of Ag (from traces to 43%) in gold and also containing Cu, Fe, Pb, less often - platinum group metals, Mn, Bi, etc. Chem. gold compounds are rare in nature, mainly these are tellurides - calaverite AuTe2, krennerite (Au,Ag)Te2, sylvanite AuAgTe4, petzite Ag3AuTe2, mutmannite (Ag,Au)Te, montbreite Au2Te3, etc. Gold is present Ch. arr. in quartz, carbonates, pyrite, arsenopyrite, galena, sphalerite, chalcopyrite. In ores, gold is present in the form of inclusions of sizes b. h. 0.1-1000 microns, sometimes nuggets up to several are found. tens of kg. Genetich. types of industrial gold deposits: hydrothermal high-temperature gold-arsenopyrite formations; hydrothermal medium-temperature quartz-sulfide and gold-quartz formations; hydrothermal low-temperature gold-silver formations; weathered and metamorphosed deposits; alluvial placers. Gold is extracted from gold ores themselves and, incidentally, from iron, copper, lead-zinc and uranium ores. World industrial gold resources (without USSR) approx. 65 thousand tons. Properties.
Gold is a yellow metal;
crystalline face-centered lattice cubic, a = 0.40786 nm, z = 4, space. Fm3m group. T. pl. 1064.4 °C, bp. 2880 °C; dense solid gold 19.32 g/cm3, liquid gold 17.22 g/cm3 (1100°C); С0p 25.39 J/(mol.K); DH0pl 12.55 kJ/mol, DH0sp 348 kJ/mol; S0298 47.40 J/(mol.K); level of temperature dependence of vapor pressure: for solid gold logp (hPa) = 3.94 - 19820/T - 0.3061gT - 0.16.10-3 T (298-1337 T), for liquid gold logp (Pa) = 10.710 + 17866/T (1337-3150 K); temperature coefficients linear expansion (5.98-19.10).10-6 K-1 (40-1200 K), volumetric (1.79-5.73).10-5 K-1 (40-1200 K); thermal conductivity 318 W/(m.K) at 273 K; r (2.06-2.84).10-8 Ohm.m (273-373 K), temperature coefficient. r 4.0.10-3 K-1 (273-373 K); diamagnetic, magnetic susceptibility -29.59.10-6. Gold is a very soft and ductile metal, Mohs hardness ~ 2.5, Brinell hardness 220-250 MPa; elastic modulus 81 GPa; srast 10-25 MPa. Gold is stable in air and water; does not interact directly with O2, H2, N2, P, Sb and C. Phosphide Au2P3 (DH0arr -102 kJ/mol) and antimonide AuSb2 (DH0arr - 13 kJ/mol) are obtained indirectly. Gold is not soluble. in solutions of alkalis and solutions, sol. in hot H2SeO4, mixtures of H2SO4 with HNO3, H2SO4 with HMnO4, as well as in aqua regia (HCl + HNO3): Au + HNO3 + 4HCl: H[AuCl4] + NO + 2H2O; after careful evaporation, yellow crystals of the complex chloroauric acid NAuCl4.3H2O are released. In aqueous solutions of cyanides (Na, Ca, K) with access to O2 or other oxidizing agents, gold is soluble. with the formation of dicyanoaurate ion (cyanidation): 2Au + 4CN- + H2O + 0.5O2: 2[Au(CN)2]- + 2OH-, which underlies the most important industrial process. method of extracting gold from ores. Gold does not interact with halogens in the absence of moisture without heating; when heated. gold powder in a halogen atmosphere, gold halides are formed. With many gold produces alloys with metals. One of the methods for extracting gold from rocks is based on the easy formation of gold amalgam. Conn. gold is unstable, hydrolyzes in aqueous solutions, and is easily reduced to metal. In table 1 shows the values of standard oxidation-reduction. potentials E0 certain connections. AuLn (where L is the ligand, n = 1.3). The most important connection Separate articles are devoted to gold [see. Gold cyanides, Organogold compounds, Potassium dicyanoaurate(I)], below is information about other gold compounds. Au(OH)3 hydroxide - dark brown crystals; when heated dehydrates with the formation first of AuO(OH), and then of sesquioxide Au2O3, which decomposes into gold and O2 above 160°C; pH in water is 2.4.10-12 mol/l at 20°C, in HNO3 solutions - up to 0.38 mol/l at 25°C, in NaOH solutions - up to 8.10-4mol/l at 25°C WITH. In the latter case, gold is present in the solution in the form of hydroxoaurate ions [Au(OH)4]- (pH 7-13). Au(OH)3 is formed by adding conc. solutions of alkali or Mg(OH)2 to solutions of H[AuCl4]. Auras are unstable and easily decompose when heated. Alkali metal aurates are well soluble. in water, the pH value increases with increasing ionic radius of the cation; Mg, Ca, Sr, Ba, Tl(I) aurates have limited solubility. Aurat with certain org. you form explosive mixtures. It is assumed that when gold hydroxide is exposed to alkali solutions, aurat anions [H2AuO3]-, [HAuO3]2-, [AuO3]3- are formed. See also table. 2. Dr. oxygen connections gold are unstable and easily form explosive mixtures. Conn. Au2O3 with ammonia Au2O3 4NH3 is called. "explosive gold"; explodes at 145°C, sometimes at lower temperatures; without explosion sol. in solutions of alkali metal cyanides. Gold hemisulfide Au2S - black-brown crystals; DG0arr 29 kJ/mol; poorly soluble in water (p-value product 4.10-69 at 25 °C), sol. in solutions of cyanides and polysulfides of alkali metals. Receive interaction. conc. solution K[Au(CN)2] with H2S from the last. heating to boiling with excess hydrochloric acid. Sesquisulfide Au2S3 - black crystals; decomposes when heated. up to 200 °C; not sol. in salt and sulfuric acid, sol. in HNO3 with the release of elemental gold, KCN solutions, bromine water. Receive interaction. H2S with AuCl3 or complex gold chlorides in anhydrous ether in the cold. Complex compounds containing the anions [AuS3]2-, [AuS2]-, [AuS]-, [Au(SO3)2]3-, [Au(S2O3)2]3- are known. AuSe monoselenide exists in two crystalline forms. modifications of the monoclinic system. When treating gold hydrochloric acid solutions in the cold with hydrogen selenide, sesquiselenide Au2Se3·H2Se is deposited, which is stable (after drying) in the range of 40-390°C; at 535-650°C it decomposes with the release of elemental gold. Selenate (IV) Au2(SeO3)3 3H2SeO3 lemon-yellow crystals; not sol. in water, sol. in salt and selenium (when heated) compounds. Selenate (VI) Au2(SeO4)3 yellow crystals; DH0rev - 954 kJ/mol; not sol. in water, decomposes with hydrochloric acid, sol. in H2SO4, HNO3 and hot conc. H2Se04. Telluride (hemitelluride) AuTe2 - crystals from brass-yellow to silver-white with metallic. shine; dense 9.3 g/cm, DH0rev - 11 kJ/mol; brittle, Mohs hardness 2.5-3. AuSCN thiocyanate is colorless. crystals; not sol. in water and org. r-retailers; at 140°C decomposes to metallic. gold and (SCN)n; under the influence of water it forms strong complex anions [Au(SCN)2]- and [Au(SCN)4]- in solutions. Colloidal gold. When restoring gold in the breakdown. solution of its salts, as well as with electric. By spraying gold in water, colloidal solutions of gold are formed, the color of which depends on the degree of dispersion of the particles, and the intensity of the color on their concentration. Gold particles in a colloidal solution are negatively charged. A hydrophobic gold sol in a hydrochloric acid aqueous solution can be represented by the following diagram: [Au]m is the core of the micelle (the number of atoms m, depending on the conditions, can vary from several hundreds to millions of units); AuCl4- are ions that determine the negative charge of the colloidal gold particle and the magnitude of the adsorption potential. layer thickness d0; H+ - counterions that determine the potential of the diffusion layer (electrokinetic potential), of which x ions are located in the blurred part of the double layer with thickness d; n is the number of AuCl4- ions adsorbed on the surface of the micelle core, with n << m. Preparation.
Sources of gold are the ores and sands of the gold alluvial and primary deposits (the gold content in them is 5-15 g/t), as well as intermediate ones.
products (0.5-3 g/t) of lead-zinc, copper, uranium and certain other products. Gravitats are extracted from gold placers. methods using the so-called traps, jigging machines, concentration resins, sluices, decomposition. flushing devices. Gold-bearing sands are mined from the bottom of rivers and lakes and enriched in dredges. When extracting gold from primary ores, a combine is used. schemes including enrichment (gravity, flotation) and metallurgical (leaching, ion-exchange sorption from pulps, cyanidation, less commonly amalgamation) operations. When using cyanidation, the crushed ore or concentrate is treated with NaCN solution with stirring; from cyanide solutions, gold is precipitated with Zn powder, using ion exchange resins or activators. coals. The final products of the scheme are usually gravity concentrate (the so-called gold head) and rough gold. Gold is purified by dissolving it in aqua regia and the afterglow. elect precipitation (for example, using FeSO4), chlorination in a melt or solution (chlorination) and electrolytic. refining in hydrochloric acid solution. Definition.
Gold is detected qualitatively by the formation of colored sediments and solutions.
They use connections. gold with Hg2Cl2, H2O2, SnCl2, K4[Fe(CN)6], KI, bsnzidine, 1-naphthylamine, o-toluidine, guaiac resin, complexone III, ascorbic acid, phenylthiourea, dithizone, rhodamine, isoquinoline, etc. You can use sorption on ion-exchange resins, as well as methods of electrophoresis, chromatography (circular thin-layer, precipitation and distribution), and luminescence. Gold is determined quantitatively gravimetrically (in the form of metallic gold), titrimetrically (by the reduction of Au3+ with subsequent titration of the excess reducing agent), photometrically (by the orange color of the bromouraate ion, as well as by the intense coloration of gold compounds with various organic reagents), electrochemically , spectral methods, activation methods, atomic absorption. and assay analyses. For pre- concentration of gold using chemicals. methods, liquid-liquid extraction and chromatography. Application.
Gold is a currency metal that serves as the universal equivalent of money.
Gold and its alloys are used for decorative purposes, making jewelry, coins, medals, dentures, and chemical parts. equipment, electrical contacts and wires, microelectronics products, for cladding pipes in the chemical industry. industry, in the production of solders, catalysts, watches, for coloring glass, making nibs for fountain pens, coating metal. surface (in aircraft construction, space technology and other areas). Art. The radioactive isotope 198Au (T1/2 2.967 days) is used to treat tumors in radiotherapy. World gold production (without the USSR) approx. 1100 t/year (1984). Basic producers - South Africa, USSR, Canada, USA, Brazil, Australia. Some Au(I) preparations are toxic and accumulate in the kidneys, and, to a lesser extent, in the liver, spleen and hypothalamus; the accumulation of gold in the kidneys can lead to kidney disease, as well as dermatitis, stomatitis, and thrombocytopenia. Gold was known to man already in ancient times; it is possible that it was the first metal that humanity began to use for its needs. There is data on the extraction of gold and the manufacture of various types of gold from it. products in Egypt (4100-3900 BC), India, Indochina (2000-1500 BC), etc. === Sp. literature for the article “GOLD” : Busev A.I., Ivanov V.M., Analytical chemistry of gold, M., 1973;
Malyshev V. M., Rumyantsev D. V., Zoloto, M., 1979; Paddefet R., Chemistry of gold, trans. from English, M., 1982; Noble metals. Handbook, ed. E. M. Savitsky, M., 1984; Marfunin A.S., History of gold, M., 1987. V.S. Strizhko, M.A. Meretukov. “GOLD” page was prepared based on materials from the chemical encyclopedia.
More on the topic:
- Gold - a guide to substances
Counterfeiting gold jewelry
If something sells well, it's a sign that something can be faked to match it. Counterfeiting of gold is perhaps one of the most common fraudulent schemes today, which is ensured by the high cost of the precious metal. Most often, “white gold” is counterfeited, passing off ordinary high-grade silver as an alloy with platinum. But an experienced buyer will always distinguish one precious metal from another.
Another chemical element that is ideal for counterfeiting gold is tungsten. Light bulb hairs, electrodes, and so on are made from it. This chemical element has a density similar to gold, so its weight is practically indistinguishable from the noble metal.
In addition, sometimes the hallmark is faked. If there are doubts about the quality of the product or if the hallmark mark does not match the hallmark number indicated in the accompanying documents, it is better to refuse to purchase such a product.
How to distinguish a fake?
There are several ways to distinguish a genuine product from a counterfeit. It should be noted that using only one test does not guarantee the accuracy of the result. It is better to check on several parameters. So, experienced jewelers use the following methods to distinguish gold from fake:
- Check softness. Real yellow metal is very soft. If you bite into it, it will leave a mark. This is exactly how you can spot a fake. Alloys with a minimum content of noble metal are hard.
- Check the sound. Real gold jewelry rings softly and easily. But you shouldn't rely on hearing alone. Different impurities distort the sound in different ways.
- They drip with iodine. If the product contains a large proportion of the desired metal, then a drop of iodine placed on the surface of the object will turn black. Otherwise, it will turn white.
- They draw on the tiles. Since the density of gold is higher than tile, it should leave a characteristic golden mark on it. If it is a fake, then the mark will remain on the product. Also, traces of gold dust will remain on the skin if the product is thoroughly rubbed, for example, on your hand.
- Weigh on special scales. If the metal density is less than declared, then the weight of the product will be less.
- Scrape the back of the object. This is done in order to find out whether ordinary copper or tungsten is hidden under a layer of gold.
Potential buyers should be wary of the following points:
- lack of documentation;
- discrepancy between the mark and the hallmark declared in the documents;
- corrections in documents;
- erased hallmark;
- hallmark of the Soviet period;
- unusual color of the product.
If something incomprehensible confuses you and there are any suspicions, this is a sure sign that you should first show the product to a professional, an appraiser or a pawnshop employee, and only then make a decision to purchase.