The quality and authenticity of a piece of jewelry is indicated by its sample. In any case, this is the opinion of most buyers. Today many people know the standard set of numbers for gold, silver and platinum. But what exactly is hidden behind these numbers and what the letter code of the name sign means is not known to everyone.
In world practice, there are four systems of samples of precious metals - metric , spool , lot and carat . Each system is tied to a specific unit of weight and has its own digital designation.
Spool sampling system
The spool sampling system was officially introduced in Russia in 1771. For silver alloys; in 1733 - for gold. In this system, the precious metal content was determined by the number of spools in one pound of alloy - 409.5 grams. Platinum and palladium products were not branded.
Zolotnik - a Russian unit of weight, about 4.266 grams, was 1/96 of a pound, i.e. 1 pound was equal to 96 spools. Pure metal corresponded to 96 samples. For gold products, 56, 72, 92 and 94 samples were provided, and for silver - 72, 74, 82, 84, 87, 88, 89, 90, 91 and 94. In Russia, this system existed until 1927, after which it replaced by the metric system of samples.
Classification of gold samples
In general, there are two main systems of samples: metric and carat, but very often on old antique things you can also find a spool sample.
Spool system
The spool sampling system was created in Russia in 1711, and was in use for more than two hundred years, until 1927. This system, like others, indicated the exact number of noble metal in the alloy; for gold, the maximum value was 96. In general, the following gold samples were established: 56, 72, 82, 92, 94.
To convert spool standard to metric, you can use the following formula:
X/96 * 1000 = Y, where X is spool standard, and Y is metric 72/96 * 1000 = 750 It turns out that 72 standard in spools = 750 gold standard
Metric system
The metric testing system replaced the spool system in 1927, and has been operating in the Russian Federation and neighboring countries to this day. There are the following metric hallmarks for gold used in Russia: 375, 500, 583, 585, 750, 958, 999. It is measured very simply - let’s say you have gold jewelry with a total weight of 1,000 grams of 585 hallmark, this means that it is pure it contains 585 grams of gold, the rest is alloying additives (copper, zinc, etc.).
Carat sample system
The karate sampling system is used mainly in the USA, Canada and some Western European countries. Therefore, questions are often asked: 18 carat gold, what grade? Or 14 karat gold, what standard?
So, the basis of the carat system is the number of carats of precious metal in a 24-karat alloy.
That is, in simple words: if you have 18 karat gold, and you want to find out how much it is in the metric system, then you need 18/24 * 1000 = 750. This means that 18 karat gold corresponds to 750 purity.
Metric system rob
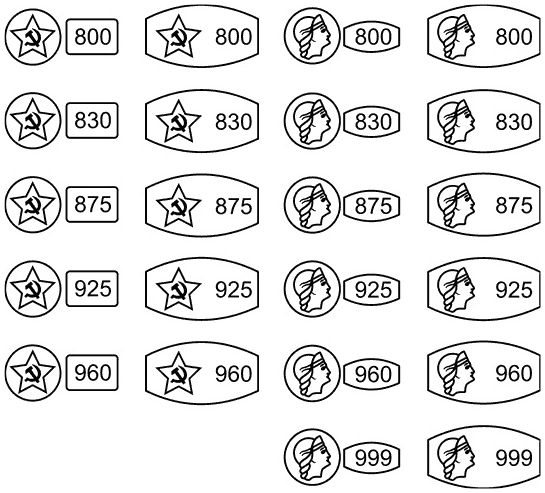
Today, this is a generally accepted international system (in force in our country since 1927). The content of pure precious metal here is determined by the number of grams in one kilogram of the alloy. Pure metal corresponds to 1000 fineness. Thus, the 585th gold standard means that there are 585 grams per 1 kg of alloy. pure gold and 415 gr. ligatures*(585+415=1000). For platinum products there are 950, 900 and 850 samples, for gold - 999, 958, 750, 585, 500 and 375 samples, for silver - 999, 960, 925, 875, 830 and 800 samples, for palladium - 850 and 500.
Carat sample system
This assay system has German roots and is intended only for gold alloys. The precious metal content here is determined by the number of carats (1 carat = 9.7 grams) in one “Cologne mark” equal to 233.8 grams. The maximum purity of pure metal is 24 carats.
This system should not be confused with the unit of measurement for the mass of precious stones, where one carat is equal to 0.2 grams.
Table Precious metal sample ratio
| Metric | Zolotnikovaya | Carat |
| 1000-999,9 | 96 | 24 |
| Silver 960 | 92 | 23 |
| Platinum 950 | 91 | 22 |
| Silver 925 | 89 | 22 |
| Silver 875 | 84 | 21 |
| Palladium 850 | 82 | 20.5 |
| Silver 830 | 77 | 19 |
| Silver 800 | 72 | 18 |
| Gold 583/585 | 56 | 14 |
| Gold, palladium 500 | 48 | 12 |
| Gold 375 | 36 | 9 |
Lot sampling system
This system was common in Germany in the Middle Ages and was used for hallmarking silver items. The content of pure precious metal was determined by the number of lots in one “Cologne mark” (233.8 grams). The maximum sample of pure silver was 16 lots. In this system, marking with Roman numerals was adopted.
Note* - Ligature - additional metals in an alloy, with the help of which the necessary physical, mechanical and aesthetic properties of the metal are achieved. Those. By adjusting the composition of the alloy, you can obtain the required hardness, ductility and other properties, and in addition the desired color of the alloy.
Lecture on chemistry “Types of samples”
Types of samples
The raw materials used are heterogeneous in composition. Therefore, taking a sample for analysis is an important part of quality control of raw materials, intermediate and final products. Failure to comply with selection rules leads to incorrect analysis results. Sampling must be carried out in strict accordance with the standards and the State Pharmacopoeia.
Depending on the purpose, samples are divided into individual, average and control.
Individual sample
is a test that characterizes the quality of a product in one container (bag, can, barrel, etc.) or at a certain level. An individual sample is taken in one go.
Average sample
consists of several individual samples taken at different levels in one or more containers. It determines the average amount of material in one or more batches and corresponds to the physical and chemical composition of the bulk of the substance. To prepare an average sample, individual samples taken are mixed well in a vessel with a capacity of 1.5-2 times the total volume of the average sample. After mixing, the average sample is transferred equally into two clean and dry vessels, thus preparing a control and arbitration sample.
Control sample
- part of an individual or average sample intended for separate analysis. The control sample stored in case of arbitration analysis is called arbitration. The arbitration sample is sealed or sealed and stored in the QCD storeroom in case of arbitration analysis during the expiration date.
The sampling method depends on the state of aggregation and the degree of homogeneity of the substance. The more homogeneous the substance, the easier it is to take an average sample. Samples of gases and miscible liquids are simply taken, and most difficult - samples of coarse-grained and lumpy solid materials. At factories, the selection of average samples and their preparation are carried out by employees of the technical control department. Laboratory technicians in workshop laboratories and machine operators must be able to take samples of the reaction mass and mother liquors.
An analytical sheet is filled out for the selected sample, which, together with the sample, is submitted to the Quality Control Department for registration in the journal, and only then the sample is sent for analysis.
Preparation of a substance for analysis consists of collecting primary and laboratory samples. A laboratory sample is taken from the primary sample by cutting it up.
Cutting is the reduction of a sample to the size necessary for analysis. It includes: grinding, mixing and reduction of the initially collected sample.
ANALYTICAL SAMPLE
ANALYTICAL SAMPLE, a part of the research object selected for analysis. It must be representative, that is, it must accurately reflect the chemical composition. composition of the object. The task of ensuring representativeness does not arise only if the object is completely homogeneous in chemical terms. composition. This condition can practically only be satisfied by well-mixed gases or liquids. Objects are usually quite diverse and vary greatly in their homogeneity. These are rocks, ore and non-metallic minerals, metallurgical products and waste. and chem. production, soil, natural water, technol. solutions and pulps, air and other gases, root vegetables, grain, hay, honey objects. and biol. research, lec. drugs, etc.
To obtain an analytical sample, a set of operations is carried out (see below), provided for by methods that differ significantly from one another depending on the object of analysis - its mass, physical. states (gases, liquids, solids, suspensions) and physical. properties (structure, density, mechanical and magnetic properties, granulometric composition, etc.), chemical. heterogeneity (change in chemical composition in space), reaction. ability, volatility of components (water, hydrocarbons, mercury), features of the analysis method used. The operations of sampling material that is in motion (moved on a conveyor belt, flowing through a pipe or chute) and stationary (lying in a stack, in dumps, in cars, or poured into a sump) differ significantly. These operations also depend on the tasks of analysis—determining the average content of one or more. components in the mass of the object, establishing the distribution of components in space (in particular, along the depth of the layer) or in time (for example, during the technological process in the reactor). The operations included in the methods depend on the required reliability of establishing the chemical. composition of the object of analysis, on the type of other tests (for metallurgical yield, for particle size distribution, for contamination with debris or magnetic materials, etc.), from technol., biol. or other requirements.
In special cases (for example, when monitoring microelectronic products), the entire analyzed object is an analytical sample. Sometimes an analytical sample is not prepared, for example. during laser probing of atm. air, x-ray radiometric analysis of ore materials under natural conditions. occurrence, continuous X-ray spectral analysis of the charge moved on a conveyor belt. But even in such cases, it is important to know and take into account which part of the object of analysis acts as an analytical sample, generating the analyte. signal, which is used to find the content of the determined component in the object.
Historical reference. Sampling techniques emerged along with assay techniques in the early Middle Ages in connection with the use of gold. Notable successes in this area were achieved in the 18th century. 19th centuries (mining schools of V.N. Tatishchev in the Urals, research by M.V. Lomonosov, work of metallurgist V.A. Lampadius in Gottingen). Exchange of information about research conducted through special services. magazines devoted to mining and metallurgy, advances in chemistry, the possibility of performing precision chemistry. analyzes of a wide variety of metallurgical products led to rapid progress and scientific generalization of sampling practices. In the end 19-beg. 20th centuries Methods were developed that are traditionally used today. In the end 20th century in connection with the widespread use of high-purity substances, the need to study the distribution of components in the depth of thin surface layers and within the cells of a living organism, to control the content of beneficial and harmful compounds. in agriculture products and food, manage fast-flowing automated technologies. processes, new approaches to the problem of sampling and their analysis have emerged. So, the hardware base automates. control systems (ACS) are automatic. devices for selection and preliminary preparation of samples, their transportation to the analyzer and preparation for analyte measurement. signal, as well as automatic analyzers based on the use of physical and physical-chemical methods of analysis. The entire complex of devices is controlled by a computer. At the same time, the use of computers allows you to create the so-called. adaptive automated control systems that continuously monitor the condition and chemistry. composition of the controlled object, changing the number and weight of samples, the time of their collection, as well as maintaining the errors of all sampling operations and their analysis within specified limits.
Basic technical operations and equipment used. When testing, the material is weighed, distinguishing between the mass of the material as delivered, the mass minus moisture and the mass minus losses during ignition (i.e. after removing adsorbed moisture and CO2, crystallized water, decomposed volatiles, e.g. hydrocarbons). The last mass needs to be known to estimate the actual mass. content of a valuable component in the supplied material.
Granulometric composition and max. The size of pieces (grains), knowledge of which is necessary when developing testing methods, is determined based on the results of sieve analysis, sifting the material through standardized sieves. In the case of fine-grained materials, sedimentation is used. analysis, air separation, optical and other methods for determining the number and size of grains.
For manual sampling, shovels (for bulk materials), forks (for shavings, hay), and tubular probes (for fine-grained materials, such as flour, grain or sand) are used. Liquid samples are taken with a pipette or special. sampling cylinders with hermetically sealed lids. These cylinders are lowered on a cable to the required depth (for example, when studying the composition of sea water) and both of their covers are automatically closed. Gas samples are taken into glass containers with drawn inlet and outlet tubes. These containers are first purged with the gas being studied for a certain time (to clear them of air), and then the tubes are sealed at both ends using a gas burner.
When taking samples from large batches of dec. For materials transported, for example, on a conveyor belt or through a pipeline, fur is widely used. samplers of various designs. Part of the flow of the tested material is continuously or periodically directed into a container, accumulating in it a combined sample of the required mass over a certain time. The so-called longitudinal and transverse sampling of point samples. In the first case, the flow of material is cut into a number of continuous strips along the flow; in accumulated capacity is allocated one or several. alternating stripes. During transverse sampling, approximately equal portions from the entire mass of the flow located opposite the cutoff are periodically cut off into the storage tank.
Fully automated systems are increasingly being used. sampling installations. For example, at large ferrous metallurgy enterprises in the melt. metal is introduced specially. device, suck approx. 100 g of melt, which hardens in the shape of a disk. The hot disk is introduced into the pneumatic mail container, where it is cooled by air flow during transportation, and fed to the automatic machine. milling machine for surface cleaning. The disk then enters the spectrometer for atomic emission analysis. Such a system serves several people at once. steel-smelting furnaces. Its work is controlled by a computer.
When preparing samples, it is important to grind and then reduce the material evenly, without loss or contamination. Grinding of small quantities of material with small brittle particles is carried out in disk grinders or mortars. Large quantities of brittle materials are crushed in jaw, cone, roller and hammer crushers, drum or ball mills (see Grinding). Metals are most often crushed by cutting, using files, as well as manual and electric. or fur. saws, cutters, drills, special cutters. forms. In all cases, cutting is carried out without emulsions or other lubricants in order to avoid distortion of information about chemical properties. composition of the material.
Mixing of the material partially occurs when forming a combined sample from point samples, when crushing the combined sample, especially when it is all placed in a crushing apparatus, and when pouring material. In addition, special mixed mixers designs.
The reduction of crushed material is carried out manually using fur. or automatic sample dividers. When manually cutting, the material is poured in the form of a cone onto a flat, clean surface, throwing each new portion onto the top of the cone so that the material is evenly scattered over its entire surface. By pressing the flat surface on the top of the cone, a flat cake is obtained. This cake is divided by straight lines into four rectangular sectors, the tops of which correspond to the top of the original cone, and the material of two opposite sectors is combined. Almost the same result is obtained using the so-called. punching cross, the edges are two vertically placed plates intersecting at an angle of 90°. The crosspiece is placed on a flat, clean surface and each new portion of material is thrown from above so that, if possible, 1/4 of the portion falls into each of the sectors of the crosspiece. The material that falls into two opposite sectors is combined.









