Precious metals, in addition to their high prices, have technical disadvantages. In their pure form, gold, silver and platinum are soft, the surface quickly loses its shine. According to experts, it is more profitable to use jewelry alloys for the production of costume jewelry, because these are expensive metals, but with additives that improve their qualities. Jewelry alloy is used to make jewelry. It does not differ in appearance from gold of various types, however, it is cheaper because it does not include expensive metals in its composition, but is only coated with them.
Jewelry alloy decoration
Description of the alloy
The most common gold jewelry alloys,
but many people do not know what it is and what elements are included in its composition
.
Their main component is gold, the content of which varies between 75–98%. The following additional components can be used: silver, copper, palladium, platinum. By adding additional metals, you can make an alloy with any shade - from red to light yellow, greenish.
Jewelry alloys are used to make expensive jewelry. They are used to make products with precious stones:
- rings;
- necklace;
- earrings;
- tiaras;
- tie clips;
- cufflinks
Ferrous metals, iron-carbon alloys with additions of chromium and nickel, are not considered jewelry, but they are used to make cheap products that imitate white gold and platinum. Stainless steel and surgical steel began to be used for the manufacture of various accessories with the advent of modern, grunge, and punk styles in youth fashion. The alloy does not darken from moisture and does not cause allergies.
Why alloy?
There are several reasons why precious metals need to be alloyed.
Strength
Precious metals such as gold and silver are too soft for practical use. Due to the fact that in their pure form they are very soft, malleable and ductile, it is necessary to improve the characteristics of metals by increasing their elasticity, rigidity and wear resistance.
Economical
The cost of pure precious metals is consistently high. By alloying precious metals with less expensive components, the cost of the final product can be reduced.
Fashion and design
Designers produce what fashion dictates. Alloying of metal to change color or physical properties, in turn, is determined by the designer's request.
Gold is the only metal of its kind that can retain its strong, unique golden hue even when doped up to 50%. Gold has become the most popular and fashionable metal ever since an ancient woman was seduced by it and a man was able to obtain jewelry for her.
Platinum is prized for its durable luster, as well as its chemical resistance and high melting point.
Status
Although governments no longer back their currencies with gold, it remains a reserve currency that is universal and accepted throughout the world.
Owning gold, be it a nugget, an ingot or jewelry, in most cultures is a tangible sign of a well-defined status of the owner. For 5,000 years, people fought and died for the possession of gold. Showing your high status with gold has always been a desirable goal.
Alloy composition
Costume jewelry is made from compounds of non-ferrous metals. Outwardly, they do not differ from gold or platinum and even repeat their shade. Such jewelry alloy requires the same care as jewelry. It is resistant to moisture, but reacts with cosmetics and iodine. According to technical characteristics, the material is durable, easy to weld and process.
The coating on all groups of jewelry alloys is made of the same metals - galvanic gold, rhodium.
In the production of jewelry alloys, in addition to gold, the following can be used:
- platinum;
- silver;
- cadmium;
- palladium;
- copper;
- zinc.
Basically, the amount of additives does not exceed 10%. The exception is silver. The greater its composition, the more plastic and lighter the alloy becomes. When the silver content exceeds 30%, gold becomes light, whitish-yellow, with a greenish tint.
The gold content in the alloy is indicated by the sample in thousandths. Fineness 916 means 91.6% gold, the rest is ligature. The numbers 999 indicate a chemically pure substance.
The composition based on copper, zinc and brass imitates gold. Depending on the proportion of components, it can be from red-red to yellow.
Tin-based jewelry alloy is widely used for making jewelry in platinum and white gold. The technology has ancient roots. The following are added as alloying substances:
- copper for ductility;
- antimony for brightness;
- aluminum for shine and strength.
Lead and nickel present in older products are prohibited by the International Standard. They cause allergies. To reduce the cost of the material, zinc is added to it.

Rings made of different metals
Alloy coating
Jewelry based on tin and non-ferrous metals is coated with a thin layer of gold, silver and platinum. The spraying thickness is thousandths of mm. As a result, costume jewelry takes on the appearance of a product made from noble materials and is protected from oxidation.
To add shine, strength, and aesthetic appearance, jewelry is coated with:
- birth;
- eloxal;
- varnish.
Gold alloy is soft, easily damaged by hard objects, and darkens from water and other substances. Platinum quickly loses its luster without a protective coating. Only rhodium is stable, hard, does not react to moisture, fats, acids and does not lose its shine over time.
The cost of rhodium is significantly higher than other noble metals. It is very hard and difficult to process. To protect products made from jewelry and gold alloys, rhodium coating is used. The coating is produced in galvanic baths.
Eloxal is sometimes used as a material for making jewelry. But more often it is used as a reliable and decorative protective coating. Sputtering of aluminum oxide - Al2O3 is first laid down in a thin layer. Then, as its thickness increases, it becomes porous. It holds paint poured into voids firmly, at the molecular level. This allows you not only to cover the product with a protective layer, but also to give it any color. After pressing, the surface begins to shine.
Varnish is mainly used to cover cheap items made from jewelry alloy and silver. The transparent layer does not allow moisture and other destructive substances to pass through and retains its shine. The coating lasts for a short time, several months, until it wears off.
Appearance
925 silver is called Sterling. It contains a ligature of 7.5% copper. Metals combine at a temperature of 894–900⁰. The alloy has the appearance of white gold or platinum, is easily processed, and takes on any shape. If desired, you can make blackening by replacing copper with germanium. The surface is smooth, shiny, and does not lose its appearance for a long time.
It is impossible to distinguish 925 silver from white gold externally. Products made from both materials are plated with rhodium, they have the same color and shine. You have to look at the mark. There is no such thing as 925 gold. If there is no marking, ask the seller for a certificate. Sterling silver is about half the price of gold.
Adding a small amount of alloy changes the properties of metals. They become more flexible and stronger. This allows you to create products with small details and filigree patterns. Externally, only a specialist can distinguish between a precious metal and a jewelry alloy.

Silver bars (Photo: pixabay.com)
Text of the book “Materials for Jewelry”
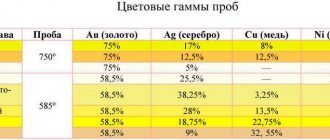
7.4. Copper alloys simulating gold and silver alloys
In order to reduce the cost of artistic products, tombac, brass, cupronickel, and nickel silver are widely used in the production of inexpensive jewelry;
in the manufacture of artistic products - bronze. Alloys of copper with zinc, aluminum, nickel, manganese, platinum and other metals have a wide range of colors. These alloys are used not only for good imitation, but also for applying decorative coating - “gold plating”. Silicon brass LK80-ZL is very popular as a gold substitute. Castings made from this alloy have a smooth surface and a beautiful golden color.
In table Figure 7.4 shows the alloys most often used when simulating 583-carat gold alloy.
Table 7.4
Chemical composition of alloys simulating gold alloys
Copper-nickel alloys with additions of zinc, aluminum, tin, lead and iron have fairly high decorative properties, imitating silver and its alloys. They can be used for casting (for example, nickel silver), for stamping (nickel silver, tombak) and drawing. Nickel silver (German: “new silver”) is most widely used for the manufacture of silver jewelry, containing, in addition to copper, 15% nickel and 20% zinc.
The chemical composition of alloys that imitate silver is given in Table 7.5.
Table 7.5
Chemical composition of alloys that imitate silver
The continuous increase in requirements for jewelry products has contributed to the creation of a number of alloys that, along with high strength, perfectly imitate silver and gold alloys (Table 7.6).
Table 7.6
Chemical composition of simulating copper-based alloys
Aluminum alloys
Aluminum alloys are classified according to manufacturing technology (wrought and cast), ability to heat treatment (hardening and non-hardening) and properties (Fig. 8.1).
Rice. 8.1. State diagram of aluminum - alloying element (diagram).
A – deformed alloys; B – casting alloys; I – non-hardening alloys and II – hardening by heat treatment.
8.1. Aluminum-based wrought alloys
Alloys that cannot be strengthened by heat treatment include the AMts and AMg alloys (Table 8.1).
The alloys are characterized by high ductility, good weldability and high corrosion resistance.
AMts alloys belong to the Al – Mn system (Fig. 8.2, a).
The structure of the AMts alloy consists of an α-solid solution and secondary precipitates of the MnAl6 phase, which transform into a solid solution with increasing temperature. In the presence of Fe, instead of MnAl6, a complex ternary phase (MnFe)Al6 is formed, which is practically insoluble in aluminum, therefore the AlMn alloy is not strengthened by heat treatment. In the annealed state, the alloy has high ductility and low strength.
Table 8.1
Chemical composition of deformable aluminum alloys
AMg alloys belong to the Al – Mg system (Fig. 8.2, b).
Magnesium forms an α-solid solution with aluminum, the concentration of which increases with increasing temperature from 1.4 to 17.4% as a result of the dissolution of the Mg2 Al3 phase. However, alloys containing up to 7% Mg give very little hardening during heat treatment.
Rice. 8.2. State diagrams: a
– Al-Mn;
b
– Al-Mg;
c
– Al-Сu.
Alloys of the AMts and AMg types are used for the manufacture of products by drawing (pen caps, pencil cases, jewelry) and welding (art products), which require high corrosion resistance.
Alloys that are strengthened by heat treatment include alloys of normal strength, high strength, etc. Typical representatives of these alloys are duralumin (marked with the letter D). They are characterized by a good combination of strength and ductility and belong to the alloys of the Al – Cu – Mg system. According to the Al – Cu phase diagram (Fig. 8.2, c)
copper and aluminum form a solid solution, the maximum concentration of copper in which is 5.65% at the eutectic temperature. With decreasing temperature, the solubility of copper decreases, reaching 0.1% at 20 °C. In this case, the θ (CuAl2) phase containing ~54.1% Cu is released from the solid solution. In alloys additionally alloyed with magnesium, in addition to the θ phase, the S phase (CuМgAl2) is also formed. The more copper contained in the alloy, the greater the amount of phase θ will be in its structure (D1). An increase in magnesium content leads to an increase in the amount of the S phase and an increase in the strength of the alloy (D16). The difference in properties is especially significant after hardening heat treatment. For example, for freshly quenched alloy D1 σв = 24–26 kg/mm2, δ = 20–22%, НВ = 60–80 kg/mm2. As a result of natural aging, Duralumin D1 acquires the following mechanical properties: σв = 38–42 kg/mm2; δ = 18%; HB = 100 kg/mm2.
When hardening, alloy D16 is heated to 495–505 °C, D1 – to 500–510 °C, then cooled in water at 40 °C. After quenching, the structure consists of a supersaturated solid solution and insoluble phases formed by impurities. With natural aging, the formation of Guinier-Preston zones rich in copper and magnesium occurs. Aging lasts 5–7 days. In the Al–Cu binary alloy, artificial aging, which consists of aging after quenching at elevated temperatures (100 °C), reduces the aging time to 1–1.2 days. With increasing aging time at temperatures of 150–200 °C, coagulation of the strengthening θ-phase (CuAl2) occurs, as a result of which the alloy softens. Thus, the process of artificial aging occurs in several stages. The first stage, as in the case of natural aging, consists of the formation of Guinier-Preston zones, which have the same nature, but are larger in size. At the second stage, over time, the zones pass into the intermediate θ-phase, and then (in the third stage) into a stable θ-phase, close to the metal compound CuAl2. In the D16 alloy, the ternary metal compound Al2CuMg (S phase) plays an important role. In this alloy, hardening during aging occurs due to the formation of zones enriched in copper and magnesium, which, when heated, transform into the intermediate phase S', which has a distorted lattice of the Al2CuMg compound. Further transition of the S' phase to the S phase (Al2CuMg) and its coagulation causes softening of the alloy. Artificial aging is not used in aluminum alloys for jewelry.
2. Aluminum-based casting alloys
Some jewelry, such as household items, smoking sets, cutlery, gun plates, elements of fountain and ballpoint pens, as well as costume jewelry, the surface of which is anodized or treated with cathode pulse bombardment (for gold), are made by casting from aluminum-silicon alloys (silumins) with high casting properties.
According to the state diagram of the Al – Si system (Fig. 8.3), silicon does not form chemical compounds with aluminum and is present in aluminum alloys in elemental form. But in its physical properties, silicon is close to chemical compounds; it has high hardness (HRC 106) and, like them, is fragile.
Despite its noticeable and variable solubility, silicon does not give aluminum the ability to be strengthened by heat treatment, which is associated with the unfavorable nature of the decomposition of the silicon solid solution in aluminum. Dissolving in aluminum, silicon somewhat strengthens it, while slightly reducing its plastic properties. An aluminum alloy containing even 10–12% Si remains quite ductile.
Rice. 8.3. AI–Si system state diagram.
Silumins are divided into double (or simple), doped only with silicon, and special ones, which in addition to silicon contain small amounts of other alloying components (Mg, Cu, Mn, Ni). Silumins are classified as eutectic or hypoeutectic alloys. Without taking into account the influence of other components (except Si), their structure is either eutectic α + Si (AL2) or primary crystals α + eutectic α + Si (AL4, AL9, ALB).
Silicon has variable solubility in aluminum, which increases from <0.1% at room temperature to 1.65% at the eutectic temperature (577 °C). Therefore, by heating aluminum-silicon alloys to a temperature close to the eutectic and rapid cooling, it is possible to obtain a supersaturated solid solution of silicon in aluminum, which, upon subsequent aging, decomposes with the release of dispersed silicon particles. However, the strengthening effect of this treatment is extremely small and has no practical significance. Thus, double (simple) silumins are among the thermally non-strengthening alloys with low strength characteristics.
The only way to slightly increase their strength and ductility is to grind eutectic silicon crystals, which can be achieved in two ways: 1) increasing the cooling rate during crystallization, 2) introducing small additives (hundredths of a percent) of alkali metals (sodium, lithium, strontium) into the alloys. . The first way gives good results. However, it has limited use in the production of thin-walled jewelry castings with fine relief details that may not be cast when casting in a metal die or during injection molding. The second way - modifying the structure of silumins with small additives - is more universal. Structure modification is usually called a change or improvement of the structure with the introduction of small additives, not as a result of the formation of any new structural components, but as a result of the influence of these additives on the size and shape of the structural components formed by other components.
In practice, modification of silumins with sodium or a mixture of its salts (60% NaF + 25% NaCl + 15% Na3AlF6 or 40% NaF + 45% NaCl + 15% NagAlF6, etc.), which are simultaneously used as refining fluxes, is widely used).
Rice. 8.4. Structure of eutectic silumin (11.7% Si): a – the alloy is not modified: b – the alloy is modified with sodium.
The introduction of 0.01% Na into Al – Si alloys leads to a sharp grinding of eutectic silicon crystals, since the sodium present in the melt during crystallization is adsorbed on the surface of the silicon crystals and prevents their further growth.
The presence of sodium in silumins causes, in addition, a shift of the eutectic point towards higher silicon concentrations, therefore alloys that were eutectic and hypereutectic before modification become hypoeutectic after modification, and dendrites of an α-solid solution appear in them instead of silicon, which become the leading phase during crystallization. In Fig. Figure 8.4 shows the structures of unmodified and modified silumin with 11.7% silicon.
In Fig. Figure 8.5 shows the effect of the cooling method during crystallization and sodium modification on the mechanical properties of binary aluminum-silicon alloys.
Rice. 8.5. Mechanical properties of AI – Si alloys depending on silicon content:
1 – modified alloy, cast in the ground, 2 – unmodified alloy, cast in the ground, 3 – unmodified alloy, cast in the chill mold.
The modification effect, i.e., the improvement in mechanical properties due to modification, is greater the higher the silicon content in the alloy, since modification changes the size and shape of silicon crystals. Modification does not have a positive effect on silumins containing less than 5% Si.
AL2 alloy is used for jewelry casting. The density of eutectic silumin AL2 is 2.66 g/cm3. It has high corrosion resistance in air atmosphere, including sea air atmosphere. Small additions of manganese and magnesium further increase corrosion resistance. The high casting properties of silumins determine their good weldability, which is important when assembling jewelry. Thermally non-strengthening eutectic silumin AL2 has high ductility, but low strength characteristics. A significant advantage of the AL2 alloy is a small crystallization interval (close to zero), so no shrinkage porosity is formed in the castings, which is very important during finishing operations - grinding and polishing of jewelry castings, since during mechanical processing the shrinkage porosity opens and deteriorates the surface of the product. In jewelry casting, these defects are not allowed.
In artistic casting, the formation of concentrated shrinkage cavities (which is typical for alloys with a short crystallization interval) causes difficulties in casting medium and complex castings. In this case, the AL4 alloy, which is hardened by heat treatment, is used, which, compared to the AL2 alloy, has a significantly smaller concentrated shrinkage cavity, which is important when casting bas-reliefs, sculptures, etc.
AL4 alloy castings are subjected to quenching and tempering. During the heating process, some enlargement of silicon particles in the eutectic occurs and transition into solution (during quenching) and precipitation (during tempering) of particles of the Mg2Si phase in a highly dispersed form, which causes additional strengthening of the alloy. AL9 alloy (Al – Si – Mg system) is often used in jewelry and art casting. The alloy contains 6–8% Si, 0.2–0.4% Mg. Before pouring the molds, it is not modified, and the casting is not artificially aged (the castings are only hardened). The alloy combines satisfactory strength, high ductility with good casting properties. In table Table 8.2 shows the compositions of aluminum alloys used in jewelry and art casting.
Table 8.2
Composition of aluminum casting alloys used in jewelry and art casting
Alloys of the second group have low linear shrinkage (1–1.4%), high fluidity β50–420 mm) and zero tendency to form hot cracks. The alloys are well processed by cutting, ground and polished well. Using the electric arc anodizing method, gold of various samples is imitated.
The technology for anodizing aluminum-based alloys is as follows. An aluminum casting with a well-prepared surface (degreased, ground and polished) and a lead cathode are placed in a cooled bath with a sulfuric acid solution (density 200–300 g/l). The process occurs at current densities of 10–50 mA per 1 cm2 of casting (required source voltage up to 50–100 V). Electrolyte temperature – up to +20 °C. The oxide film formed at elevated temperatures is colorless, which allows it to be painted with any dyes. At low temperatures, the film turns golden (gold-like).
The electric arc method, called ion bombardment condensation (IBC), uses a vacuum chamber in which a cathode is placed (Fig. 8.6). As a result of the applied voltage, an electric arc occurs between the chamber body and the cathode. Ions, electrons and neutral particles fly out from the cathode spot. Some of these particles fall on the product located inside the chamber. First, the particles loosen the surface layer of the product, effectively cleaning it and heating it to 300–500 °C. Next, the surface layer is saturated with atoms of the material from which the cathode is made. If nitrogen is introduced into the chamber, a nitride coating is formed on the surface of the product.
Figure 8.6. Scheme for applying coatings using the CIB method:
1 – cathode; 2 – neutral particles; 3 – electrons; 4 – ions; 5 – casting.
Titanium nitride coatings, which successfully imitate gold plating, have become widespread. Moreover, by adjusting the process parameters, it is possible to achieve complete resemblance to gold of various samples. Such coatings are characterized by strong adhesion to the product material and high wear resistance. When applying coatings using the CIB method, very stringent requirements are imposed on the quality of the surface of products: it should not be contaminated, such as rust, oil and other non-metallic materials.
Zinc-based alloys
Zinc is widely used to make jewelry. Fasteners, zippers, buttons, elements of inexpensive beads, decorative overlays for gift folders and guns, personal jewelry (chains), jewelry boxes, etc. are made of zinc alloys. In its pure form, zinc is used for anti-corrosion and decorative coatings.
The mechanical properties of zinc are characterized by the following average indicators: σв = 15 kg/mm2, δ = 20%, ψ = 70% and НВ = 30 kg/mm2.
Zinc-based alloys are used to produce artistic and jewelry castings by injection molding and die casting.
According to the state diagram of the Zn – Al system shown in Fig. 9.1, the primary crystals are a solid solution of aluminum in zinc (β-phase), and the eutectic is a mixture of α and β crystals (α-solid solution of zinc in aluminum). However, upon slow cooling, the α-phase at a temperature of 270 °C decomposes to form a eutectoid: α → α1 + β, where α1 is also a solid solution of zinc in aluminum, but contains 27% Zn. Phase α contains: 79% Zn and 21% Al.
It is necessary to point out that if by rapid cooling it is possible to prevent the decomposition of an α-solid solution, then, due to its unstable state at ordinary temperatures, decomposition occurs already in finished products. This process is often called natural aging. With aging, the properties and linear dimensions of castings change. The latter is typical for zinc-magnesium alloys, but in zinc-aluminum and zinc-copper alloys no change in linear dimensions is observed. A double alloy of zinc and aluminum has the structure shown in Fig. 9.2.
Rice. 9.1. Zn-AI phase diagram.
Rice. 9.2.
Microstructure of zinc alloy with 4% aluminum. Visible are light primary crystals of the β-solid solution and the α + β eutectic (dark field).
Among other alloys for jewelry and art casting, it is worth highlighting the binary zinc-copper alloy and the ternary alloy for injection molding, containing 4% Al, 3% Cu, 0.1% Mg, and the rest Zn. The Zn – Cu alloy compares favorably with the Zn – Al alloy in that aging is not observed in it with the same casting properties. However, it has lower mechanical properties. Ternary zinc alloy for die casting with 4% Al, 3% Cu, 0.1% Mg has the highest mechanical properties and best casting properties compared to other zinc alloys.
Solders
Zinc solders are mainly used for soldering aluminum alloys. Solder, which is an alloy of zinc with 40% cadmium, has proven itself well. This alloy has a melting point of 266 °C, a tensile strength of 100 MPa and an elongation of 5%.
Solder is a hypereutectic alloy of the cadmium-zinc system, the structure of which consists of primary crystals of a P-solid solution of cadmium in zinc and eutectic (α + β), where α is a solid solution of zinc in cadmium. The state diagram is shown in Fig. 9.3.
Rice. 9.3. Phase diagram of Cd – Zn.
Silver and its alloys
Silver is a chemical element, a metal. Atomic number 47, atomic weight 107.8. Density 10.5 g/cm3. The crystal lattice is face-centered cubic (fcc). Melting point 963 °C, boiling point 2865 °C. Brinell hardness 16.7.
Silver is a white metal. It is considered the second noble metal after gold. Polished pure silver practically does not change its color in air. However, under the influence of hydrogen sulfide in the air, a dark coating forms on the surface over time - silver sulfide Ag2S. Silver, compared to gold and platinum, is less stable in acids and alkalis.
Silver deforms beautifully in both cold and hot states. It polishes well and has high reflectivity.
The widespread use of silver in photography and electrical engineering is due to its unique physical properties - the highest electrical and thermal conductivity among metals.
Despite the fact that silver is a relatively rare element (its content in the earth's crust is only 7 x 10-6%, in sea water it is even less - 3 x 10-8%), it has been widely used in jewelry production for many centuries. This is primarily due to the high decorative properties of silver, as well as its unique ductility. Silver jewelry is often made using the filigree technique, a pattern made from thin wire. Threads for silver embroidery are made from silver.
For the manufacture of jewelry, as well as in the electronics industry, both pure silver and its alloys with copper and platinum are used.
Grades of silver and silver alloys are regulated by GOST 6836-80.
The standard applies to alloys intended for electrical conductors and contacts, jewelry, and strings of musical instruments.
According to this standard, silver alloys are designated by the letters Ср, followed by alloys (Pt - platinum, Pd - palladium, M - copper). The numbers after the letter designation of the alloy indicate the mass fraction of silver, expressed in ppm (tenths of a percent) for pure silver and silver-copper alloys (for example, Sr999, SrM91b, SrM950, etc.), or the mass fraction of the main alloying components, expressed in percent (in this case, the number is separated from the letter designation not by a space, but by a hyphen, for example: SrPl-12 (12% Pt, 88% Ag), SrPd-40 (40% Pd, 60% Ag).
All silver alloys (GOST 6836-80) can be used in the electrical industry for the production of contact groups for various purposes. For the manufacture of strings of musical instruments, the alloy SPM 950 is used.
GOST 6836-80 establishes grades of silver and silver alloys with copper, platinum and palladium, intended for the manufacture of semi-finished products by casting, hot and cold deformation. Other silver alloys are regulated by industry standards or specifications.
The chemical composition of silver and its alloys must comply with the standards specified in tables 10.1, 10.2, 10.3 (GOST 6836-80). Silver-platinum alloys, being more expensive, are used less frequently in the jewelry industry.
Table 10.1
Silver
Table 10.2
Silver-copper alloys
Table 10.3
Silver-platinum alloys.
Varieties
Jewelry alloy contains various components. For the manufacture of costume jewelry and other items, the following is usually used:
- bronze;
- brass;
- cupronickel;
- pewter;
- nickel silver.
Bronze is a compound of copper and zinc. It has a reddish red gold color. To impart various qualities, it is alloyed with aluminum, beryllium, silicon, and phosphorus.
Bronze is an alloy of two components of copper and zinc without additives. To produce the material, pure copper and galmey, zinc ore, are used. It is characterized by high strength and hardness.
Cupronickel is a complex alloy that contains non-ferrous metal:
- copper;
- manganese;
- iron;
- nickel.
Looks like silver. It is characterized by high hardness and strength. Requires constant care as it gets dark quickly.
Pewter is a tin-based alloy. The soft and pliable material is easily coated with silver and gold.
Nickel silver, a three-component alloy, consists of: zinc, copper, nickel. It is resistant to moisture and has a beautiful yellowish tint.

Bronze jewelry
Shiny sterling silver
For many years people have been seeking silver that will not tarnish, but it is only in the last 5-6 years that there has been a breakthrough with real improvements in the qualities of standard sterling silver. Until recently, only one standard sterling silver alloy was generally used: 92.5% silver and 7.5% copper, the qualities and characteristics of which are well known. A beautiful, white, shiny, decorative metal, easy to make and cast, but will immediately develop scale when using conventional soldering and annealing methods. When polishing, if the process is not controlled, an unpleasant black tint may appear on the metal surface.
We now have formulations that improve hardness and minimize finishing times with non-stick properties and improved tarnish resistance.
In the early 80's I tried an alloy containing silicon and zinc, but found it too soft and did not continue development. A little later, while attending a symposium in Santa Fe, I was offered a formula for an oxidation-resistant silver alloy. It certainly proved resistant to oxidation in the flame of a torch, but it was also too soft to be used in jewelry making.
It was necessary to give this alloy a certain degree of hardness. Therefore, to increase hardness, it was decided to introduce reasonable amounts of germanium.
Initially, the ligature contained seven elements. It took a lot of experimentation to balance all the characteristics, since a change in the concentration of components even less than 0.1% can significantly change the result. By carefully adding mainly germanium, zinc, silicon and trace elements, we have been able to develop a range of sterling silver alloys.
We have patented silver alloys of 830, 925 and 950 samples.
Where is it used?
Bronze is in demand in industry for the manufacture of gaskets, fittings, and decorative door handles. Cups and dishes are cast from it, and elements for window grilles and railings are made. Some countries use coins made of bronze.
Gears, decorative elements for furniture facades, taps, and O-rings are made from brass. From jewelry :
chains, rings, earrings, bracelets and other accessories.
Cutlery, dishes, jugs, and dishes are made from cupronickel. Jewelry is often made in silver.
Replicas of items made from precious metals and various costume jewelry coated with precious metals are made from pewter alloys.
Medals and other awards are stamped from nickel silver. Alloys are used for the manufacture of parts of medical equipment and precision instruments. Nickel silver is used to make jewelry, guitar frets, and tableware. Often confused with cupronickel.
Rafting inspiration
When a metal with certain properties is required, a decision may be made to create a new alloy if, after market research, it is determined that this is not available among the many commercial alloys offered.
It's easy to take a few ingredients, melt them together in a crucible and see what happens, but it takes a little smart planning to achieve the desired result. For example, should the final alloy be hard or soft? Will it fuse and harden quickly? What color should the alloy be? What other special qualities are needed for a particular use?
Ideally, the alloy is required to be easily processed using conventional jewelry making techniques, and can also be easily polished to produce a shiny surface in the piece.
The basic properties of the alloy must comply with the standards for precious metals established by tradition and supported by the legislation of most countries of the world.
The known and proven qualities of metals and elements previously used to produce similar alloys should be studied. Even with all these options, there is still a very wide range of elements that can be drawn with once the desired properties are achieved.
Advantages and disadvantages
As a result of combining various materials, the jewelry alloy acquires the necessary qualities. The appearance of the products allows you to produce jewelry with an expensive look at a budget price. Alloys made of non-ferrous metals are in demand in industry.
The main disadvantage of products made from jewelry alloys is the fragility of the coating. You need to handle rings and chains with care and constantly look after them. When the thin film wears off, the material begins to oxidize and deteriorate.
Silver alloys
In silver alloys, copper, zinc, cadmium, aluminum and nickel are used as alloys.

Characteristics of silver alloys:
- an alloy of silver and copper can have a color from white to red-copper depending on the percentage of metals, combines strength with ductility;
- the alloy of silver with zinc is white in color and lends itself well to jewelry processing;
- an alloy of silver and cadmium, white in color, hard, with a cadmium content of more than 50 percent, it becomes brittle;
- an alloy of silver with aluminum has a light gray color and is ductile if the aluminum content in the alloy is no more than 6 percent;
- an alloy of silver with copper and cadmium, white in color, lends itself well to jewelry processing.
Platinum assay systems
Similar to gold hallmarks, three types of hallmarks are also used for platinum: metric, spool and carat. The spool test was used for jewelry only in Russia, and was replaced by metric after 1927. In Western Europe and the USA, it is customary to use the carat platinum hallmark. All types of hallmarks can be easily converted from one to another - more about this in the article on hallmarks of gold alloys. The table below shows the correspondence between samples of different types for platinum: