Chemical composition
Chemically pure gold is almost never found in nature. Usually contains impurities of silver (Ag), copper (Cu), and less often other metals, with which it forms solid solutions.
The most common impurities are Ag, Cu, Fe, Te, Se, less often Bi, Pt, Ir, Rd (with a significant content of impurities, varieties of gold are distinguished). Mg, As, Mn, Ni are determined spectroscopically. Primary gold from low-temperature deposits is generally richer in silver than gold from high-temperature deposits; Secondary gold (redeposited) is poor in silver.
Varieties of gold
Electrum - electrum - (Au, Ag). Contains up to 10-15% Ag. Named from the Greek - amber - due to the similarity of color (Pliny). Synonym for silver gold. It is observed in the form of grains, plates, lumps, dendrites, and sometimes crystals. Predominant forms: (111), (100). Doubles by (111). There is no cleavage. The fracture is hooked. Very malleable and viscous. Hardness 2-3. Density 12.5—15.6. The color is light yellow to silver-white and greenish ("green" gold from Baley). The streak is metallic and shiny. Opaque. Good conductor of electricity. The reflectivity is very high, but lower than that of gold and silver. Isotropic. HNO3 is poisoned (it dims slightly from the vapors, sometimes boils slightly); from KCN it darkens with the formation of a rough surface; from FeCl3 it sometimes turns slightly brown and iridescent, from HgCl2 it becomes brown to black and iridescent. The Ag content in electrum is above 25%, usually 30-45%. Melting point is 1061° with an Ag content of 35.4% and 1046° with an Ag content of 39.9%. More rare than native gold and silver. Found in medium to low temperature deposits. Compared to native gold, poor silver has a lower temperature.
The largest number of indications of electrum finds relate to quartz, barite-quartz, calcite-quartz and barite veins and deposits. Mineral secretions have different sizes and shapes. Associated with argentite, red silver ores, stromeyerite, galena, chalcocite, stibnite, native gold and other minerals (Valei in the Chita region, Kochkarskoye deposit in the Chelyabinsk region, Zmeinogorskoye, Zyryanovskoye and other deposits of Rudny Altai). In some sulfide deposits, electrum is observed in the zone of sooty ores or in the oxidation zone (Kryukovskoye, Petrovskoye, Ridderskoye, Semenovskoye and other deposits of Rudny Altai). Small grains and nuggets of electrum weighing up to 400 g are found in some placers (Altai, Western and Eastern Siberia). In the oxidation zone it changes with the formation of films and crusts of silver halides on the surface.
Porpecite - porpezite (Froebel, 1892) (palladite) - Au, Pd. Installed for the first time in 1798 in the Porpets (Pompeo) region in the state. Minas Gerais in Brazil. Forms irregular spongy secretions, films, plates, rounded nodules, and less often crystals. The fracture is uneven, hooked. Hardness—3. Forging. Density 15.73. Metallic shine. The color is like gold itself, partly with a grayish or bronze tint; sometimes silver-white. The line is metallic and shiny. Under artificial conditions, Au and Pd give solid solutions in all proportions. Known in pcs. Minas Sherais and Goias in Brazil in primary deposits and placers. In primary deposits, porpecite is confined to the so-called yacotings - contact rocks occurring among itabirites (layered quartzites with hematite). Yakotings are observed near outcrops of granite rocks; consist of dolomite, calcite, chondrodite, tourmaline, pyroxene, actinolite, magnetite, cassiterite. Porpecite was found in small quantities in placers in the river basin. Chorokh (Georgia). The formation of porpecite during the alteration of earthy palladite (PdO) has been established. In appearance, porpecite is indistinguishable from gold. When mercury cyanide is added to a nitrate solution, a white gelatinous precipitate of palladium cyanide precipitates.
Cuproauride (Loyasechkin, 1939) and auricupride (Ramdor, 1950) are natural solid solutions of Ag and Cu, similar in composition to AuCu3 (cuprous gold). Under a microscope, in most cases it turns out to consist of two components: cuprous gold and golden copper. The study of the Au-Cu system showed that homogeneous isomorphic mixtures (Au and Cu) decompose with decreasing temperature to form AuCu and AuCu3. The nature of the secretions is no different from gold that does not contain copper. The color is yellowish-pink. The fracture is hooked. Forging. Hardness 2-3. It amalgamates weaker than pure gold, is etched more strongly than high-grade gold, oxidizes when heated to 260°, and when heated for a long time at 350° for 3 hours. cuprous gold powder turns black and becomes covered with thin films of CuO. The main and most studied deposit of cuprous gold is Mount Karabash (Chelyabinsk region), where cuprous gold is accompanied by magnetite, chalcocite, argentite, breuthauptite and gold-bearing native copper; gold-bearing quartz veins occur in the tectonic zone among serpentinites. The weight of accumulations of cuprous gold reaches 100 g, but along with large secretions, its microscopic secretions are widespread. Etching with KCN reveals lamellar and lattice structures of solid solution decomposition. The Cu content is 17–20%. In the Urals, cuprous gold is also found in some placers (Mountain Shield in the Sverdlovsk region). In addition, cuprous gold has been discovered in primary deposits in Australia, Northern Finland, and South Africa.
Bismuthaurite (bismuth gold) - bismuth gold (Au, Bi). At a Bi content of up to 4% it is homogeneous (solid solution); at higher contents, inclusions of native bismuth are detected under a microscope. In terms of the shape of the secretions and properties, it does not differ significantly from gold itself. Typical samples come from the Shilovo-Isetskoye deposit in the Sverdlovsk region (quartz veins with bismuth gold, tetradymite, pyrite, chalcopyrite, fahlore). Transitions from bismuth gold to maldonite containing about 35% Bi are not known.
Will give birth -rhodite (Adam, 1869) (born). Contains up to 43% Rh. Possibly represents a mixture. Density. 15.5-16.8. Fragile. Found in deposits in Mexico and Colombia. In Russia, it was found in gold-bearing placers in the Chorokhi River basin in Georgia (with a content of 11.6% Rh).
Iridic gold (iraurite) - iridic gold. Found in placers in the Chorokhi River basin (Georgia) and in California (USA). Density 21.69. Possibly represents a mixture. Composition of iridistigo gold from the Chorokh River basin: Au - 62.1; Ag - 2.1; Pt - 3.8; Ir - 30.4; Pd - word; Cu - 0.03; Fe - 0.6; Bi - traces.
Platinum gold - platinum gold. Found in placers in the Chorokhi River basin (Georgia). Density 19.53. Composition: Au - 84.6; Ag - 2.9; Pt - 10.5; Cu - 0.9; Fe - 0.2. Possibly a mixture.
Crystallographic characteristics
Syngony. Cubic.
Class. Hexoctahedral.
Crystal structure
Main forms : a(100), d(110), o(111) e(210), m(311), x(10.18.1), t(421).
general characteristics
There are primary deposits (including veins with a gold content of 1...30 g/t) and placers in the form of alluvium (gold content of 0.5...50 g/m³).
In addition to gold-bearing ores themselves, gold-containing ores of copper, nickel, lead and zinc, silver, iron (ferruginous quartzites), and manganese, in which gold acts as an associated component, are known. More than 30 gold minerals have been discovered. The main industrial importance is native gold, secondary - kustelite (Au about 10-20%) and tellurides: calaverite - AuTe2 (40-43% Au), krennerite - (Au, Ag)Te2 (40% Au), sylvanite - (Au , Ag)Te4, (25-27% Au), petzite Ag3AuTe2 (25% Au). Rare - cuproauride - AuCu2, rhodite - Au, Rh, porpecite - Au, Pd, aurostibite AuSb2, maldonite Au2Bi, gold sulfide uytenbogardeite - Ag3AuS2, etc. Associated components of gold ores - Ag, Cu, Pb, Zn, Bi, As, Sb , Te, Hg, W, Sn, Co, Ni. There are endogenous, exogenous and metamorphosed gold ores.
Endogenous gold ores
All endogenous gold ores are of hydrothermal origin. The Au content ranges from 2-3 to several hundred g/t. They form massive plate-like, saddle-shaped veins, pipe-like bodies, veinlet and stockwork deposits.
Rich gold-quartz ore
The composition of gold-bearing ores is varied (up to 200 minerals). Gold-sulfide-quartz ores predominate. Calcium and iron carbonates, barite, chlorite, sericite, and tourmaline are present. Among the ore minerals, pyrite predominates, and arsenopyrite is less common. They are accompanied by pyrrhotite, sulfides and sulfosalts of copper, lead, zinc, bismuth, silver, iron oxides, native silver, bismuth, and in some cases tellurides.
Exogenous gold ores
Exogenous gold ores are concentrated in placers, less often in oxidation zones of gold-bearing sulfide deposits. Gold is found in the form of rolled and semi-rolled grains, flakes (size 0.5-4 mm), sometimes intergrowths with quartz in sand or clayey material containing boulders, pebbles and (or) crushed stone of various types. There are nuggets. Au content - from 100-150 mg/m³ to tens of g/m³, fineness - from 800 to 950. In oxidation zones, gold is concentrated in the lower parts of oxidized ores, mainly in association with iron and manganese hydroxides, with supergene minerals of copper, arsenic, silver, carbonates, kaolinite. Au content - from 2-3 to 10 g/t. Gold-bearing ores form complex deposits, lenses and nests.
Metamorphosed gold ores
Metamorphosed gold ores are associated with layers of gold-bearing conglomerates, less often - gravelites. Gold in the form of grains, sometimes semi-rounded (5-100 microns), is laid in quartz-sericite-chlorite cement, as well as in the form of thin veins that intersect quartz pebbles. Au content 3-20 g/t, fineness above 900.
Extraction of gold ores
The total amount of gold extracted from the bowels of the Earth in the historically foreseeable period, according to experts, exceeds 135 thousand tons. Moreover, more than 40% of this amount is represented by jewelry, 30% is concentrated in state reserves, almost 20% is in the form of bars and coins , and only 10% is used by industry for technical and technological purposes.
At the end of the 20th century, it became profitable to process poor and difficult-to-process ores: to include off-balance reserves into exploitation; to resume the operation of previously “mothballed” quarries and landfills, mines and shafts; process man-made waste from many mining and processing plants. Fundamental changes have occurred in the technology of beneficiation of gold-bearing ores due to heap, as well as heap with cyanization and biological leaching in columns, the “coal in pulp” method, and the improvement of other pyro- and hydrometallurgical methods (for example, autoclave beneficiation of refractory ores). This led to an increase in the profitability of secondary processing of low-grade ores and tailings from processing plants with a gold content of 1.0-0.3 g/t or less.
A sharp reduction in direct costs and overall losses in gold production was facilitated by the rapid transition from underground to open-pit mining (over the period from 1988 to 2003, the share of open-pit mining increased in the world from 30 to 70%) and the active introduction of highly productive equipment in mining operations, when transporting and processing ore.
World gold production in 2009 amounted to 2,572 tons. Largest producers:
- South Africa (220 t. (2008),
- USA (298 tons (2002),
- Australia (225 t. (2009),
- Indonesia (90 t (2008),
- China (313.98 tons (2009),
- Russia (205.2 tons (2009),
- Canada (95 t. (2009),
- Peru (175 tons (2008),
- Uzbekistan (85 t. (2001),
- Ghana (72 volumes (2001).
Beneficiation of gold ores
The enrichment process is a single system in which individual elements are interconnected. It is possible to achieve high results only taking into account a systematic approach, which takes into account the interaction of system elements, that is, in this case, a full range of processes.
Gravity enrichment is undoubtedly one of the most well-known processes. It is to him that history owes the fact that gold was the first metal with which humanity became acquainted several thousand years BC. Nature itself took care of this, freeing gold deposits from the minerals that contained them in the beds of rivers and streams flowing through gold-bearing rocks, giving them such an attractiveness that our distant ancestors could not help but pay attention to. Mass extraction of gold from placers began with gravitational enrichment methods, after which these methods actively “stepped” into the factory technology of processing ores from primary deposits.
Schemes and modes of enrichment of gold ores significantly depend on their mineral composition, destruction, the presence or absence of impurities that complicate the extraction of gold, as well as on the size of gold particles.
Low sulfide bedrock ores
Depending on the size, gold is usually extracted from low-sulfide primary ores using a one- or two-stage gravity-flotation scheme in combination with amalgamation or cyanization. If the ore contains sufficiently large gold, then after the first stage of crushing, gravity enrichment is used. This scheme using gravitational processes allows you to extract up to 80% of gold.
When cyanidating gravity enrichment waste, gold recovery increases to 95%. However, cyanization is unacceptable for ores that contain carbonaceous substances, as well as copper and antimony sulfides. In addition, cyanidation does not extract gold, which is finely embedded in sulfide minerals. In this case, it is advisable to use flotation of gold together with sulfide minerals. With small and uneven inclusions of sulfides and gold, better results can be obtained by beneficiation using staged flotation schemes. However, in the case of receiving waste with a gold content higher than the dump one, they are subjected to gravity enrichment in hydrocyclones or in jigging machines with the return of the sand fraction or concentrate to the beginning of the process or to an independent cyanidation cycle.
Gold-pyrite ores
In gold-pyrite ores, fine gold is usually associated with pyrite, so it is isolated by flotation together with pyrite. To obtain waste with waste gold content, the control flotation front is extended to obtain a finished concentrate in each control operation, which is sent for cyanization. If gold finely disseminated in pyrites is not extracted by cyanization, the flotation concentrate before cyanization is burned at a temperature of 650 - 700 ° C to obtain a porous undercooked substance, which ensures the opening of gold grains. Sometimes, to reduce the loss of gold from waste, cyanidation is used. However, if there is free gold in the ore, during burning it is absorbed by the fusible components of the ore and is not recovered during further cyanidation. In this case, a scheme is used in which the gravity concentrate is subjected to cyanidation with the dissolution of free gold. Cyanization waste is sent to sulfide flotation with further burning and cyanization of the concentrate.
Sulfide gold-copper ores
In sulfide gold-copper ores, gold is not only in a free state, but also finely disseminated in sulfides (mainly chalcopyrite). In addition to copper sulfides, ores usually contain pyrite, arsenopyrite, and pyrrhotite, which also contain gold, but in smaller quantities than chalcopyrite. Such ores, after removing free gold from them by gravitational processes (jigging, beneficiation on sluices) and grinding to a particle size of 70% class - 0.2 mm, are sent to the 1st collective flotation, where xanthate and pine oil are fed. After grinding the flotation waste to a particle size of 95% class - 0.2 mm, free gold is removed from it by jigging, and the classification is discharged to the second collective flotation, which is also carried out with xanthate and pine oil.
The collective concentrate after refining operations is sent to copper flotation, where pyrite is depressed with lime, but at low alkalinity, because gold is depressed in a highly alkaline environment. The resulting gold-copper concentrate, after dehydration and drying, is sent to a copper smelter. During the electrolytic processing of blister copper, which is created during smelting, noble metals go into electrolytic sludge, from which noble metals are extracted at special plants. The pyrite concentrate is sent for cyanidation to extract the gold contained in it. The total gold recovery using this scheme is 90–91%.
Gold-arsenic ores
Gold-arsenic (gold-arsenic) ores are the heaviest beneficiation object, because they can contain up to 10% arsenic in the form of arsenopyrite with a significant amount of thin, almost emulsified inclusions of gold. In addition to arsenopyrite, the ores usually contain chalcopyrite. These ores are very difficult to enrich due to the presence of carbonaceous shales (refractory ores).
Enrichment of gold-arsenic ores is carried out using a combined gravity-flotation scheme. After separation from the original ore by jigging with purification on gravity concentrate concentration tables, the waste from the gravity cycle is sent to flotation with the release of a collective concentrate.
Particularly difficult during the flotation of sulfides are carbonaceous substances that go into the concentrate and significantly increase their yield, but reduce the gold content. In addition, these concentrates cannot be further processed by cyanidation, because carbonaceous shales are a sorbent of the gold-cyanide complex. In this case, the carbonaceous concentrate from the collective concentrate is separated with the addition of lime, foaming agent and kerosene, and the flotation waste of carbonaceous shale with the addition of copper sulfate is divided into gold-pyrite and gold-arsenic concentrates.
Polymetallic ores
In polymetallic ores, gold is usually found in a finely dispersed state in sulfide minerals, primarily in pyrite and chalcopyrite, less commonly in galena and sphalerite, and, in addition, can be in a free state.
The technology for extracting gold from polymetallic ores consists of capturing free gold in a grinding cycle and more completely extracting it with concentrates in which it is associated with the main valuable components.
Gravity enrichment of gold ores
Currently, gravitational concentration of gold is widely used at gold extraction factories in all countries of the world, including those that are the main producers of this metal.
Based on the nature of the processed raw materials, these factories are divided into 3 groups:
- quartz and quartz-sulfide ores containing noble metals mainly in cyanide-soluble form.
- cyanidation-resistant pyrite and arsenic-pyrite ores with finely disseminated gold in sulfides, as well as ores containing sorption-active carbonaceous matter.
- complex ores containing, along with gold and silver, heavy non-ferrous metals (copper, lead, zinc, antimony), as well as uranium.
Within each group, the number of enterprises using the processes of gravity, flotation enrichment and cyanidation is determined (Tables 1, 2).
Table 1. Scope of application of gravity, flotation and cyanidation
| Name indicators | Groups of enterprises | |||
| Simple ore | Persistent ore | Complex ore | Total | |
| Total number of enterprises | 142 | 53 | 44 | 239 |
| Including the number of enterprises using: | ||||
| gravity | 42 | 17 | 19 | 78 |
| flotation | 26 | 36 | 43 | 106 |
| cyanidation | 137 | 47 | 25 | 209 |
Table 2. Gravity enrichment of ores
| Name indicators | Groups of enterprises | |||
| Simple ores | Refractory ores | Complex ores | Total | |
| Number of enterprises using gravity enrichment | 42 | 17 | 19 | 78 |
| Including: as the only technological process | 1 | — | — | 1 |
| in combination with cyanidation | 23 | — | — | 23 |
| in combination with flotation (without cyanidation) | 2 | 3 | 5 | 10 |
| in combination with flotation enrichment and cyanidation | 16 | 14 | 14 | 44 |
Gravity enrichment is practiced by more than 1/3 of enterprises, but gravity is almost never used without combination with other processes.
In recent years, great progress has been made in the technology of gravity enrichment of gold ore raw materials. This is manifested, first of all, in the creation of new devices capable of extracting not only large, but also very small particles of metallic gold, released during the ore grinding process, such as centrifugal concentrators and centrifugal jigging machines, in which the intensity of separation of gold particles and other minerals is less grain density increases many times.
In the vast majority of cases, gravity is used in combination with cyanidation, flotation, or both. For Simple ores, the most typical schemes are gravity and gravity-flotation enrichment with cyanidation of flotation tailings, and in some cases, gravity concentrates. The main purpose of gravity in these options is to remove large free gold from the ore into products (concentrates) that are processed in a metallurgical cycle separate from the bulk of the ore.
In addition to increasing (usually 2-4% of total gold recovery), this makes it possible to prevent or at least significantly reduce the accumulation of gold in grinding and mixing apparatus.
Flotation, like gravitational enrichment, refers to mechanical enrichment methods, when the concentration and separation of mineral components is carried out without disturbing their crystal structure and chemical composition. Such methods may also include magnetic, electrical and radiometric separation (including photometric sorting), separation of minerals by shape and particle size, selective adhesion (collection by sticky surfaces) and some other processes. However, unlike the methods listed above, including gravity ones, flotation is based on the use of chemical reagents that perform a variety of functions.
The basis of flotation enrichment, carried out, as a rule, in an aqueous environment, is the principle of imparting hydrophobic properties to the grains of the extracted component, due to which they are not wetted by water and are “pushed” to the boundary of the liquid and gas phases, even if the density of these grains is many times higher than the density water.
Hydrophobicity is imparted to mineral grains by collector reagents (collectors) introduced into the suspension and fixed on the surface of the extracted particles, for example sulfides. The process of separating the latter from the rest of the ore mass (“tailings” of flotation) is intensified by aerating the pulp with air, using special foaming agents and reagents that depress the flotation of gangue minerals, as well as by regulating the pH value, i.e. creating an acidic, alkaline or neutral pulp environment.
Thanks to the extremely wide range of flotation reagents, the total number of which is about 6-8 thousand, it is possible to concentrate virtually any minerals by flotation. On the same basis, principles and methods of separation (selection) of various mineral mixtures have been developed to obtain individual products (concentrates) that meet market requirements and the conditions for their subsequent use or chemical and metallurgical processing. In this regard, flotation, as a method of mechanical enrichment of mineral raw materials, has very great potential, which determines its widespread use in various industries, including non-ferrous and ferrous metallurgy, the coal industry, in the production of diamonds, graphite phosphorus, barite, magnesite, pure koalin clays and other mineral products. Currently, more than 2 billion tons of minerals are processed annually by flotation, and this is the best characteristic of this technological process.
Flotation plays a fairly important role in the beneficiation of gold ore raw materials. However, this takes into account one important circumstance that distinguishes the flotation capabilities of gold ores from most non-ferrous metal ores. The latter are characterized by a clear division of the main technological stages: ore dressing and metallurgical processing of concentrates. These stages are carried out at individual enterprises (concentration plants, metallurgical plants), which are often part of various production associations. At the same time, the overwhelming majority of gold extraction factories operate according to schemes with a complete cycle of ore processing to the final marketable product - gold bars (Dore alloy). For this reason, ore processing at gold mining enterprises is usually carried out using combined schemes, combining gravity-flotation enrichment operations with cyanidation and other chemical and metallurgical operations (smelting, roasting, autoclave or biochemical oxidation, etc.).
Flotation beneficiation of ores at gold recovery factories
| The name of indicators | Groups of enterprises | |||
| Simple ores | Refractory ores | Complex ores | Total | |
| Total number of enterprises analyzed | 142 | 53 | 44 | 239 |
| Of these, flotation enrichment is used | 26 | 36 | 43 | 105 |
| including: as the only technological process | — | 3 | 13 | 16 |
| in combination with cyanidation and gravity | 26 | 33 | 30 | 89 |
According to flotation activity in ores, gold-containing minerals can be arranged in the following sequence (in descending order):
- intergrowths of metallic gold with iron sulfides (pyrite, arsenopyrite) and sulfides of heavy non-ferrous metals (chalcopyrite, galena, etc.);
- actual gold-bearing sulfides, in which gold is present in the form of thin metallic inclusions;
- free grains of gold and natural alloys of gold and silver (electrum, kustelite, etc.)
The greatest effect from the use of flotation is achieved when extracting gold from ores with predominantly sulfide mineralization. Flotation is used extremely rarely for oxidized gold ores, since it does not provide satisfactory recovery of metal into concentrates, being much inferior in this regard to the process of direct cyanidation of ore. However, the use of flotation turns out to be very useful in the process of mineralogical studies for isolating fine grains of free gold from oxidized ores for their subsequent microscopic examination in order to establish the size and morphology of gold grains. As a rule, the flotation process of gold ores is carried out in a slightly alkaline environment (pH = 7-9). To create such an environment, soda or lime is used (the latter is used less frequently, since it has a weak depressive property in relation to gold-bearing pyrite, and to some extent, to native gold).
Ethyl or butyl xanthates are used as collectors. Pine oil or cresol is usually used as a foaming agent. To activate pyrite, copper sulfate is fed into the pulp (during grinding).
Depression of gangue minerals, including clays, is produced by silicate and (less commonly) sodium sulfide. The latter is also used for sulfidation of the surface of particles of oxidized minerals (malachite, azurite, cerussite, anglesite, smithsonite, etc.) in order to impart flotation activity to them.
For the flotation of gold and silver ores, depending on their material composition, a variety of devices are used: multi-chamber mechanical, pneumomechanical, pneumatic, as well as large-volume (tank) flotation machines. In recent years, flotation columns have been developed and are successfully operating at a number of gold mining enterprises, designed for the enrichment of finely ground and slurry ores for the concentration of native gold and coarse gold-containing sulfides in ore grinding cycles. Flash flotation is seen as an alternative to gravity methods for extracting gold from “freshly crushed” ores and is effectively used in factories.
Flotation is used extremely rarely as the only technological process. These are mainly enterprises processing complex ores, which, along with gold and silver, contain other non-ferrous metals (copper, lead, zinc, antimony) in concentrations and mineral forms that allow the possibility and economic feasibility of the associated extraction of these metals into liquid marketable products. Carrying out flotation in a special reagent mode makes it possible to isolate copper, lead, zinc and antimony concentrates of standard composition from gold ores, which are sent for subsequent processing to specialized metallurgical plants. During flotation, a significant portion of the noble metals present in the feedstock also passes into these concentrates. The possibilities of their subsequent extraction are determined by the technology of the main metallurgical production.
The main strategy of gold mining enterprises carrying out complex processing of polymetallic ores, in addition to obtaining quality concentrates of non-ferrous metals through flotation, is to ensure the maximum possible extraction of gold on site using other technological processes, in particular gravity enrichment and cyanidation. This kind of combined gravity-flotation-cyanide technology for processing complex ores is practiced by most enterprises.
Favorable objects for the use of flotation are technologically resistant ores, in which gold is closely associated with iron sulfides and cannot be extracted by cyanidation without the use of rather complex and expensive preparatory processes: oxidative roasting, autoclave or biochemical oxidation of sulfides.
Flotation allows not only to concentrate gold-containing sulfides (pyrite, arsenopyrite) in a small volume of concentrate sent for metallurgical processing, but also to separate these sulfides, for example, pyrite and arsenopyrite or pyrites of various generations, differing in gold content.
As one of the options, the enrichment of low-grade gold ores (Au 2.2 g/t) occurs using a combined gravity-flotation technology. In the flotation process, a special activator of metallic gold and gold intergrowths with pyrite is used. In combination with potassium amyl xanthate (pyrite collector) and carbon dioxide introduced into the pulp to maintain the optimal pH value = 8.4-8.6, the reagent makes it possible to extract 85% of gold into the concentrate while retaining about 75% of the pyrite present in the flotation tailings mainly fractions that do not contain gold. Taking into account gravity, the total recovery of gold into concentrates at the plant is more than 90% - with a concentrate yield of only 1.9% of ore.
When processing carbonaceous sulfide ores, improving the quality and reducing the yield of gold-containing concentrates is achieved through preliminary flotation removal of waste coal fractions from the ore in terms of gold content, or through sequential flotation of carbon and sulfides with careful selection of the reagent regime at each stage.
If there is a simultaneous presence of refractory (in sulfides) and easily cyanidated gold in ores, flotation enrichment is supplemented with a cyanidation operation, which is subjected to either the original ores before flotation or the tailings of flotation enrichment. The pyrite and arseno-pyrite concentrates obtained during flotation are also processed on site by cyanidation, but only after preliminary chemical, thermochemical or biochemical exposure of gold-bearing sulfides.
At enterprises processing ores of simple composition with relatively easily cyanidated gold, flotation is used only if it ensures the production of waste tailings containing gold and if at the same time the costs of hydrometallurgical processing are significantly reduced, since not the entire mass of ore is subjected to cyanidation, but only flotation concentrates.
Flotation has become an extremely diverse process in terms of the reagents used and the equipment used, which makes it possible to use it much more widely than before, including on low-grade and complex ores. Due to flotation, it is possible to increase gold recovery and ensure acceptable profitability of deposit development. At the same time, the multivariate nature of the process requires versatile and thorough laboratory and technological studies of ores, as well as extensive experience and knowledge, in order to find exactly the option that will provide the best effect for specific conditions.
The basis of modern technology for extracting gold and silver from ores of primary deposits is cyanidation, which consists of selective leaching of noble metals with aqueous solutions of alkaline cyanides: sodium, potassium, calcium. Then the dissolved metals are separated from the solutions by various methods to ultimately obtain high-quality commercial products - metal ingots (Dore metal), sent to refineries. In some cases, the refining of gold and silver is carried out directly on site, i.e. in the conditions of a gold mining enterprise.
It should be noted that in previous times, cyanidation of gravity concentrates containing large particles of gold and other heavy minerals (in particular sulfides) in tank-type devices (mechanical and pneumomechanical agitators) was considered unacceptable due to the low rate of dissolution of gold and the difficulties of maintaining the suspension in suspension. condition, which resulted in the settling of heavy fractions at the bottom of the apparatus. Currently, these problems are being solved through the use of horizontal drum mixers, as well as devices with forced circulation of cyanide solutions and cone reactors. These devices allow cyanidation of gold-containing gravity concentrates with almost any granulometric characteristics. Thus, the traditional technology of gravitational concentration of gold with deep finishing of primary concentrates to rich “gold heads”, suitable for smelting into gold-silver alloy (Doré metal), is complemented by an alternative method of hydrometallurgical processing of concentrates with moderate metal content, after their one- or two-time refining on concentration tables or other finishing devices.
The effectiveness of this option increases even more if not only gravity concentrates are subjected to cyanidation, but also tailings from gravity enrichment of ore (using a “softer” leaching mode), since in this case it is possible to direct the solid residues of the “concentrate” cycle into the general hydrometallurgical process with ultimately obtaining a single commercial product.
The history of the world mining and metallurgical industry, most likely, does not know other examples of such dynamic development and mastery of technological processes, such as cyanide leaching of gold. This is evidenced, for example, by the following figures. The cyanidation process was patented in October 1887. The following year, 1888, a demonstration semi-industrial installation was created, and in 1889, the world's first factory for cyanidation of gold ores was built. A year later, the second industrial cyanidation plant came into operation, the production of gold in which in 4 years increased from 9 kg (1890) to 9 tons (1893), i.e. a thousand times. The subsequent rapid development of cyanidation technology led to the fact that this process very quickly took a leading place in the overall world production of gold from ore raw materials, which over 110 years (1890-2000) grew from 200 to 2500 tons per year. Over the past 20 years, 92% of the world's gold has been obtained from the ores of primary deposits using cyanidation (the remaining 8% is the share of metal extracted along the way from the ores of heavy non-ferrous metals: copper, lead, antimony, etc.).
The technological advantages of cyanidation, carried out using solutions with a very low concentration of cyanide (0.3-1 g/l and below), lie, first of all, in the fact that it is carried out in a slightly alkaline environment (pH = 9.5 ~ 11.5) at normal (“room”) temperature and atmospheric pressure, which determines the high economic efficiency of cyanidation of gold ores.
An important role was played by the developments of the US Bureau of Mine (US BM) on the adsorption extraction of gold from cyanide media with granular activated carbons (1952) and heap cyanide leaching (HC) of lump ores and ore dumps (1969).
The first commercial carbon adsorption gold heap leaching facility was established in 1974 for rock dumps containing less than 2.5 g/t gold, which at that time made it unprofitable to process them using conventional mill technology. In the 1980s, the KB process became extremely widespread in the gold mining industry in the United States, and then in other countries. This was facilitated by the next development of USBM for the preliminary agglomeration of finely crushed and slimy ores before KB (1979). In Russia, over the past 10 years, about 20 industrial enterprises have been created that carry out heap leaching of gold ore raw materials, with a total processing volume of more than 5 million tons per year.
As a rule, open-pit ores with a gold content of 0.5 to 1.5 g/t are subjected to heap leaching, from which 50 to 80% of the metal is recovered by cyanidation. This ensures the profitable operation of enterprises of various sizes: from 0.5 to 15 million tons of ore per year. Sometimes combinations of heap and dam ore leaching operations are used.
The bulk of the ore is subjected to heap leaching after preliminary crushing to 65 mm and agglomeration of the crushed ore with lime and cyanide solution. Processing of low-grade ores (Au less than 0.5 g/t) is carried out without crushing and agglomeration using the dam leaching method. The recovery of gold into solutions is 70%, incl. 80% - with heap leaching and 65% - with dam leaching.
Another direction for increasing the efficiency of the hydrometallurgical process is the integration of heap and dam leaching operations with factory cyanidation technology.
The dam leaching process is carried out on ore of “bottom-hole” size without preliminary crushing. Gold is extracted from solutions in a separate installation. Gold-saturated coals from both leaching cycles are combined and eluted using standard technology. The total gold recovery is 90%, including in the factory technology cycle - 95% and in dam leaching - 73%.
The possibility of cost-effective processing of low-grade gold ore materials by cyanidation is confirmed by the practice of enterprises that carry out additional extraction of gold from stale enrichment tailings of previous years. This issue, given its significance (including for the Russian gold mining industry), deserves special consideration in a separate publication. Here it should only be noted that taking into account the minimal costs for the development of this type of “technological” gold deposits and the preparation of stale tailings for subsequent hydrometallurgical processing (cyanidation using factory technology), the profitability of the process is ensured when gold recovery is at the level of 0.4-0.5 g/ t of feedstock.
The objects of cyanidation application are not only poor, but also fairly rich gold-containing materials, in particular, concentrates from flotation and gravity concentration of ores.
As for gravity gold-containing concentrates, until recently the only acceptable method of processing them was considered to be deep finishing (cleaning) followed by smelting the resulting “gold heads” into metal ingots. However, special devices have now been created that allow large grains of metallic gold to be leached with cyanide solutions.
An important area of use of the cyanide process is the processing of refractory ores and concentrates. These include materials containing dispersed inclusions of gold in dense and cyanide-insoluble grains of iron sulfides: pyrite and arsenopyrite. The possibility of processing such materials using “cyanide-free” hydro- or pyrometallurgical methods has been studied for a long time. But no positive results were obtained from an economic point of view. Therefore, almost all currently operating gold mining enterprises extract gold from refractory pyrite and arsene-pyrite ores (concentrates) using the same cyanide process, but only after additional mechanical (fine and ultra-fine grinding), chemical (autoclave oxidation), thermochemical (roasting) or biochemical opening of gold-bearing sulfides. As a rule, these preparatory operations cost much more than cyanidation itself. However, collectively they provide high gold recovery into the final marketable product and the overall economic efficiency of the technological process.
Cyanidation also plays a significant role in the processing of complex gold ores containing copper, lead, antimony, zinc and other heavy non-ferrous metals, the associated extraction of which seems technologically possible and economically feasible.
Form of being in nature
Veins in quartz, dendrites, small nugget and spot gold Magadan region Utinskoe deposit
The appearance of crystals . Crystals of octahedral, less often dodecahedral and even less often cubic appearance, etc. Crystals are usually distorted: lamellar along (111), elongated along a 3rd order axis, sometimes tetragonal in appearance due to uneven development along the quadruple axis.
Twins at (111) are simple and more complex. Skeletal, parallel and stepped accretion, crystalline skeletons, dendrites and reticulate plates are observed. The dendritic structure is also found in single-crystal formations during etching. The surfaces of the edges are often uneven, with patterns of growth and erosion. Some of the deposits consist of alternating zones of varying colors - from dark yellow to silver-white, which probably depends on changes in the chemical composition of gold during its deposition.
Aggregates. Primary gold (root and placer) is observed in the form of grains, flakes, leaves of various shapes and sizes, in solid masses - nuggets of various shapes, weighing from several grams to tens of kilograms. Less often, it is also found in the form of plates, tree-like, thread-like, mesh-like, wire-like formations, dendrites, rarely in distorted, even more rarely in more or less regular crystals. Finely dispersed “invisible” gold with particle sizes of several microns or less in the form of mechanical inclusions is scattered in sulfides (pyrite, galena, chalcopyrite, arsenopyrite, etc.). Secondary gold is observed more often in the form of films, spongy deposits, rims, fine hair-like deposits on primary gold, as well as on supergene minerals (for example, cuprite, limonite, etc.). Secondary fine-crystalline gold with a brown color (similar to the color of dry mustard powder) and a dense or loose composition is sometimes called mustard gold.
Additional devices
A minidrag is a device that is used in underwater searches. In terms of operation, it resembles a regular vacuum cleaner, comes in different sizes and, accordingly, has different productivity. Small mini-dredges weighing 24 kg are capable of processing 100 kg of material per hour, and large devices weighing 90 kg can process 1000 kg/hour.
A minidrag consists of an engine, a pump, an injector, a flushing chute, a floating system on which all the equipment will be located and sometimes the air supply system will be turned on.
The minidrag sucks material from the river bottom into the injector, which then falls into a washing trough that separates the gold from other materials. Searching for gold in this way is not always easy or simple, because there are a large number of stones, boulders and other obstacles along the way.
The second device that is used in searching for precious metals is a gold probe. This is an electronic device that detects gold on soil and under water. It consists of a probe, at the end of which there is a sensor device, and a control unit is installed on the probe handle. They look for gold in the soil by sticking a probe into the ground, the sensor comes into contact with the metal and produces a certain signal, after which the light turns on. The magnetite probe reacts with a different sound tone and light bulb color. It catches those small gold particles that are inaccessible to a metal detector.
Physical properties of gold
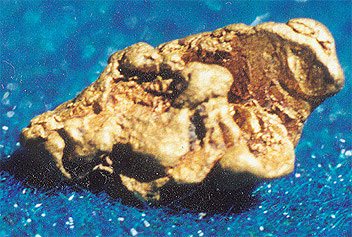
Small nugget. Utinskoye field
Optical
- Color. Color and feature, depending on Ag content, from golden yellow to almost silver white; with Cu content it acquires a pinkish tint.
- The color of the fine powder is brown.
- The shine is strong metallic.
- Transparency. Opaque; in the thinnest layers there is blue and green translucency.
Mechanical
- Hardness 2-3. When fired and remelted, the hardness decreases.
- Density 15.6-18.3 depending on composition.
- There is no cleavage.
- The fracture is hooked.
Chemical properties
High-grade gold is soluble in aqua regia. It interacts with at the moment of its release and, due to this, dissolves in mixtures in which free Cl is formed, for example, in a mixture of hydrochloric and chromic acids. Also dissolves in H2SO4, in hot concentrated. H2SO4 with an admixture of HNO3, in H2SO4 with an admixture of HJO3 or KMnO4. Insoluble in HCl and HNO3. Dissolves in cyanide solutions. In polished sections from KCN it turns black, and the structural features of gold grains are revealed. With Hg it gives a white amalgam; sometimes the HJ gets corroded. Aqua regia, as well as a solution of CrO3 in HCl and CrO3 in aqua regia reveals the structure; granular structure, twins, sometimes dendritic structure of grains; in cuprous and silvery varieties - decomposition structures.
Other properties
Malleable and malleable. Easily flattens into thin leaves. There have been cases of magnetism in native gold containing Fe. Electrical conductivity is less than that of silver and copper. Heating behavior. Melting point 1062.6°.
Home techniques
Industrial gold mining differs significantly from private gold mining, and the methods used have many advantages. If you know how to identify gold in a stone, you can successfully remove it from there using the most primitive methods.
And you don’t have to go to distant Siberia in search of abandoned mines. You can implement the idea of withdrawing precious metals at home. Back in the days of the Soviet Union, skilled craftsmen extracted it from old watches and other yellow metal products. In the recent past, gold plating was present on many radio components and household appliances, which was justified by its practicality and resistance to corrosion. Without a doubt, the percentage of gold content in such objects is negligible, but in the rock it is not that high.

If you want to get the precious yellow material at home, just take an old wristwatch and then fill it with nitric acid in glass or plastic containers. The substance will quickly dissolve any materials except gold. The resulting solution must be carefully passed through several layers of gauze, and the seized gold must be infused in vodka for 24 hours. This will result in a brownish tint.
In the future, it is necessary to rinse the composition with plain water, filter again and put on low heat to melt. To improve the efficiency of the latter process, soda is added to the gold. We must not forget about the ability of the yellow metal to evaporate, but through melting it is cleared of excess impurities and becomes like a tiny ingot.
Gold can also be extracted from radio components , in which it was used back in the Soviet Union due to its inertness and minimal electrical conductivity. To implement such an idea, you need to use “regia vodka”, which consists of nitric and hydrochloric acid. Such a substance can dissolve gold at room temperature. By the way, there are historical facts about the dissolution of gold medals in order to hide them from the fascist military. Under the influence of aqua regia, gold precipitates, after which it is thoroughly filtered and washed.
Artificial acquisition
It is obtained in many ways, for example: 1) by precipitation from gold solutions with iron sulfate, hydrogen peroxide, sulfurous acid, amyl alcohol, etc.; 2) electrolysis. The precipitation of gold from solutions with natural sulfides (pyrite, galena, sphalerite, chalcopyrite), calcite, siderite, etc. at different temperatures was artificially reproduced. Co-precipitation of gold and sulfides from solutions has been studied.
Diagnostic signs
Similar minerals. Can be confused with chalcopyrite, pyrite, millerite.
The main diagnostic features of gold: golden yellow color, low hardness (easily cut with a knife), high malleability and density, inability to oxidize in air. In small segregations, gold can be mixed with pyrite, chalcopyrite, and millerite. It differs from them in less hardness, lack of tarnish, and malleability. In polished sections under a microscope, it is easily distinguished from chalcopyrite in reflectivity and in its reaction with AgNO3 (does not turn black); differs from native silver in its greater reflectivity in blue light by blackening from KCN; from tellurides - isotropy.
Associated minerals. Quartz, sulfides, chalcedony, calcite, ankerite, siderite, rhodochrosite, adularia, barite, fluorite, zeolites, magnetite, zircon, cassiterite, ilmenite, platinum and osmic iridium.

In quartz
Origin of gold
Gold can be indigenous (vein) and alluvial. J. London expressively described the indigenous gold deposit: “... We examined the gold-bearing vein, which stood out on the rock, like a real vein on the human body...” Native gold is released from thermal aqueous solutions associated in origin with acidic and intermediate magmas, rarely with basic magmas. Characteristic of hypothermal, mesothermal and epithermal veins. This type of gold is called vein gold. In addition, there are placer gold deposits (placer or schlich gold), formed as a result of the destruction of primary (vein) deposits by river flows or sea surf. Most of the mineral is mined from placer deposits. 1000 tons of earth rock contains only 5 g of gold, and in rich gold ores there is the same amount of gold per 50 kg of rock.
Model of the formation of a jasperoid type gold deposit:
Origin and location
Hydrothermal in quartz veins (vein gold). Due to its chemical resistance, gold accumulates in placers (placer gold) in the form of rounded or flattened grains and nuggets.
It is found in minute secretions in igneous, sedimentary and metamorphic rocks. Occasionally found in pegmatites. Concentrated in hydrothermal deposits of various formations, mainly associated with acidic (to medium) intrusive, less often effusive rocks. Gold deposits are either primary or alluvial. The distribution of gold in primary deposits is uneven; Often areas are found that are sharply enriched in gold (“bushes”, “pillars” and “bonanza”).
Mineral Change
In the oxidation zone, finely dispersed gold under special conditions (exposure to Cl, Br or J at the time of separation, exposure to Fe2(SO4)3 in the presence of atmospheric oxygen, transition to a colloidal solution) dissolves - migrates and redeposits. The gold that makes up the larger segregations undergoes a change in the oxidation zone and in placers with the formation of thin rims of higher grade gold of a bright yellow color along the periphery and along the boundaries of the grains; Due to electrochemical corrosion, gold goes into solution along with more easily soluble silver, and part of the gold is immediately deposited right there on the rest of the gold. When gold remains in placers for a long time, the gold grains are refined with the removal of silver and the formation of high-fineness, relatively wide rims near the unchanged core. When transported in placers, gold undergoes mechanical abrasion and rounding, external and internal deformation and recrystallization.

Veins of gold in quartz. Magadan region, Utinskoye field
History of origin and theory of the appearance of precious gold
Perhaps, noble gold has been considered the most valuable rock on planet Earth since time immemorial, because this rare and non-renewable resource of nature, which gradually entered human use, truly became known in the Iron Age of the Bronze Age.

When the ancient people of the ancient era of the golden age of the Greco-Roman civilization of medieval Mesopotamia believed that the round placers of native pieces of alluvial deposits of sparkling gold were accidentally found:
- on solid ground
- or in the sunny valley of a flat river,
created by the bright sun using the unique power of natural water.
Already in ancient times of the 4th century AD, the Holy Bible of the canonized scriptures of religious Christians briefly mentions the golden gifts brought by wise wise men from the Middle East to the pious baby who was born into the world, who later became the spiritual teacher Jesus Christ.
The name Aurum of the useful substance of gold from Latin means: the pre-dawn breeze, where the mythological deity of the morning dawn - the all-powerful Aurora on a winged chariot with a golden-colored solar halo above her head brought daylight from the heavenly stars to the sinful earth to all good people and almighty gods.
The modern theory of the origin of valuable gold claims that it appeared in the solar system under the influence of a nuclear explosion from the destruction of neutron stars.
Where, as a consequence, some of it condensed on the newly formed molten surface of the Earth, which is why most of the metal fossil is located in the hot core, which passed through the intermediate layer of the hot mantle. However, the amount of useful gold that is located in the earth's crust was undoubtedly delivered to our planet in prehistoric times by the heavy bombardment of celestial bodies from cosmic asteroids, due to which the metallic rock ore spread over the entire surface of the earth, originating long before our era.
And now the Inca Empire collected the largest reserves of gold:
- for the cladding of temple complexes with precious plant fruits and the construction of a statue of the Sun God,
- for the production of household items, valuable bowls with decorated caskets and luxurious dishes with drinking vessels, a 700 meter chain and cash coins,
- for a military ransom and the creation of an altar throne in the form of a bloody altar chair,
where the molten metal of precious yellow gold was poured into the open throat during the death ceremony of the ritual sacrifice of an innocent being as a gift to the deity.
The basis of the cult belief of the highly developed civilization of the American continent of the pre-Columbian era was the widespread shedding of the blood of a pure being in the form of a man who, due to the fall of his ancestors, was able to please the holy spirit through a mortal test and thereby achieve final reconciliation with the divine creator, the Lord God.
Place of Birth
Among primary gold deposits, veined medium- to high-temperature deposits, in which ore bodies are often mainly or almost entirely composed of quartz, are widespread. Some deposits of this group represent stockworks and silicification zones, mainly among metamorphic and igneous rocks. Deposits of this group are usually associated with large massifs of granitoids. The ores contain certain amounts of pyrite, arsenopyrite, less often galena, sphalerite, chalcopyrite, marcasite, fahlores, wolframite, molybdenite, pyrrhotite, tellurides, scheelite, etc., non-metallic minerals include barite, carbonates, chlorites, sericite, tourmaline, garnet , apatite, albite, etc. Gold is observed in irregularly shaped grains, plates, dendrite deposits, and sometimes in crystals. Some of it is represented by thin inclusions in sulfide and other minerals. Gold flakes and grains in sulfides come in various shapes: flattened, spheroidal, rod-shaped, etc. In some deposits, large continuous segregations (nuggets) are found. Sometimes fine gold is installed only chemically. Examples of deposits of this group in Russia are many deposits of the Urals ( Berezovskoye , etc.), in Kazakhstan (Stepnyak, etc.); in the USA, a number of deposits in Alaska, California , Nevada; some deposits in Brazil, Canada, South Africa, Australia and many others. In addition to quartz veins, gold deposited from high- or medium-temperature hydrothermal solutions is also observed locally among skarns and serpentinites. A significant part of the gold is confined to low-temperature hydrothermal deposits, which are typical for areas of development of Tertiary volcanic rocks. Vein minerals are represented by quartz, chalcedony, calcite, ankerite, siderite, rhodochrosite, adularia, barite, fluorite, and zeolites. Gold in some deposits is finely dispersed in quartz, chalcedony, and carbonates. Among the ore minerals associated with it are pyrite, galena, sphalerite, chalcopyrite, fahlores, enargite, native silver, argentite, and telluric compounds of gold (nagiagite, calaverite, etc.). The composition of gold is predominantly low grade. Examples of deposits of this type: Baley (Chita region); Cripple Creek in Colorado (USA), Pachuca ( Mexico ), deposits in Japan, Romania, etc.
Placer gold deposits, modern and ancient (Paleozoic, Mesozoic), are widespread. There are eluvial, alluvial and marine placers. Their formation is associated with the destruction of gold-bearing veins and gold-bearing rocks. Gold is represented by grains and flakes of various sizes and shapes, varying degrees of roundness - depending on the type of placer and the distance from the primary deposits; There are also crystals, their intergrowths, dendrites, nuggets weighing from grams to units and tens of kilograms. Gold is usually of primary origin, released during the destruction of veins; redeposited secondary gold is also observed in small quantities, which forms rims on primary gold, copper, cuprite, and platinum. Companions of gold in placers: magnetite, zircon, cassiterite, ilmenite, platinum and osmic iridium. In placers of river valleys and terraces, gold is distributed unevenly or in “streams” with a certain pattern. Examples: eluvial placers - Kalgoorlie in Western Australia; channel placers—Aldan, Amur, Kolyma (Russia), Alaska, California (USA); terrace placers— Aldan, Lena, Okhotsk coast (Russia); marine - Alaska (USA). The largest gold deposits of the auriferous conglomerate type are metamorphosed ancient placer deposits exposed to hydrothermal solutions (Witwatersrand in South Africa). In the oxidation zone of sulfide deposits, residual primary gold , released during the destruction of sulfides, and in more rare cases, secondary (supergene) gold released from solutions. Such gold in some deposits such as pyrite deposits is observed among brown iron ores or jarosite (Maikain in Kazakhstan). Secondary (supergene) gold in the oxidation zone is usually represented by films, small crystals and their intergrowths, sometimes so-called mustard gold. Some supergene gold is formed during the weathering of gold tellurides. Gold nuggets sometimes reach large sizes: a nugget weighing about 153 kg was found in placers in Chile, weighing 93.5 kg in the Hill End quartz vein in New South Wales (Australia). A large nugget (weighing about 36.04 kg) was found in 1842 in one of the mines in the Miass region (Chelyabinsk region); in the same placer nuggets weighing 10.08 kg (1826), 20.07 kg (1854) were found; in the river placer Tyelgi, a tributary of the Miass, in 1936 nuggets weighing 14 kg 231 g and 9 kg 386 g were mined; in the Sverdlovsk region, in the placer of the Nikolsky Log, a tributary of the Chusovaya, a gold nugget weighing 13.8 kg was found (1935).
Useful mining and classification of deposits of the most valuable gold
Since ancient times, primitive people have accidentally found golden pebbles in the yellow sand and at the bottom of running streams, surprising them with the bright shine of the sun. Even then they began to understand the unique value and amazing rarity of the gold metal.

Humanity began to meaningfully mine useful gold since the Copper Age, where 5 thousand years ago the ancient state of Egypt, located in the Nile Valley in the historical territory of Nubia, became the first gold miner.
The ancient Assyrians, in their possession of gold, mercilessly conquered their closest neighbors. The indomitable thirst for possession of the precious metal forced the ancient Greco-Roman rulers to mercilessly plunder countries and force captive slaves to work tirelessly in gold mines.
Already in the New World, clear traces of useful metal mining were discovered, where the Aztec civilization and the Indian Mayans had countless reserves of divine gold. The Peruvian Incas also engaged in useful gold mining.
With the advent of white people and their discovery of shiny nuggets on the stone bottom of clear streams, a world-famous madness began - the gold rush, where predatory gold mining by almost a million people in newly discovered gold deposits lasted for almost 200 years. And now, global gold mining with a comprehensive base of natural gold raw materials began to use 2 main types of deposits:
- primary - radical
- and secondary - alluvial,
where, according to the amount of gold reserves, primary deposits are divided into the following groups:
- unique - over 1000 tons,
- very large - from 100 to 1000 tons,
- large – from 100 to 400 tons,
- medium - from 25 to 100 tons,
- small – less than 25 tons,
and accordingly, according to the amount of gold reserves, secondary deposits are divided into the following groups:
- unique – over 50 tons,
- very large - from 5 to 50 tons,
- large – from 1 to 5 tons,
- medium – from 500kg to 1 ton,
- small – less than 500 kg.

Nowadays, placer deposits of mineral gold reserves are quite depleted, but in Russia alone, gold mining from geological deposits accounts for almost half of all production of the valuable metal.
Crystal optical properties in thin preparations (sections)
In polished sections, in reflected light, golden-yellow, shiny. Reflectivity. exceptionally high, depends on the Ag content, for pure gold (in%): for green rays 47.0, for orange 82.5, for red 86. Isotropic. The refractive index (according to Kundt) in prisms for red light is 0.38, for white light 0.58, for blue light 1.00; in reflected light (according to Drude) for Na light 0.366, for red 0.306; the absorption coefficient for Na light is 7.71, for red light 10.2. The ability to polish in small deposits is very good; in larger deposits, even with thorough polishing, scratches from the smallest particles of abrasives and small pinholes remain. 'Structures of replacement and cementation of various minerals with gold are observed.
Applications of gold
Gold is a currency and monetary metal; In addition, it is used as decoration and luxury items, as well as in dentistry and gilding of metals. Gold has such unique properties as exceptional anti-corrosion and chemical resistance, high electrical and thermal conductivity. Gold is used in jet engines, rockets, spacecraft, nuclear reactors, supersonic aircraft, in the electronics and radio engineering industries, in the production of chronometers, galvanometers and equipment for the production of synthetic fabrics.
Modern microelectronics cannot do without gold—it is used in electronic computers. Gold salts are used in photography (tinting). Used in chemistry, medicine (in the treatment of some forms of tuberculosis, lungs, larynx, skin, eyes) and for coloring glass and porcelain red.