Curious readers, hello. I’ll start with a riddle: “What is harder than gold and heavier than steel?” That's right, we're talking about noble silver. I will tell you why it is so important to know the density of silver in this article. The review contains the main characteristics of this rare element of the periodic table, useful information on testing and accessible tips for determining density.
Metal in nature
More than 6 thousand years ago, the first silver products began to be made in the Middle East. And when alloyed with gold, it served as the material for the world’s first coins. And for many centuries it has been valued by jewelers for its high quality, ease of processing and appearance.
Found in nature in nuggets. The largest nugget to date was found in Canada. Its length was 30 meters, and when melted down, 20 tons of metal were obtained. Unfortunately, the chemical activity of this metal allows it to be found only in the form of compounds, often they are silver salts, which contain selenium, sulfur, or other chemical elements. Metal reserves in the world today amount to about half a million tons, and the largest quantities are mined in Peru, China, Mexico, Australia and Chile.
Electrum
The alloy consisting of silver and gold is called electrum, which means “amber” in ancient Greek. Electrum is a mineral that is a type of gold nugget. Scientific and technological progress has allowed humanity to produce electrum in industrial conditions.
The alloy of silver and gold consists of at least 50% argentum. Electrum was popular several centuries ago. Such an alloy is a suitable raw material for the production of metal money - coins. Electrum money was durable because silver gives gold its strength.
Gold and silver alloy rings
An alloy of gold and silver was also used in the manufacture of jewelry, decorative items and cutlery. Such products were characterized by high quality and wear resistance, and all due to the fact that the alloy combines the characteristics of both silver and gold. These are strength, anti-corrosion properties and lack of reactions when interacting with other chemicals.
The jewelry industry uses various gold alloys, ranging from 375 purity, where 37.5% is gold and the rest is alloy, to 750 purity alloy, where gold accounts for 75%. Can such alloys be considered electrum? The answer to this question will be negative, since only an alloy that is more than half composed of silver can be called electrum.
Properties of noble metal and its alloys
In its pure form, silver is a refractory, very ductile metal. From one kilogram you can get a wire 2 km long. It does not oxidize under the influence of oxygen, which is why it is classified as a noble metal. However, it interacts with sulfur and iodine, which form a dark film on the surface, which is why silver jewelry requires maintenance.
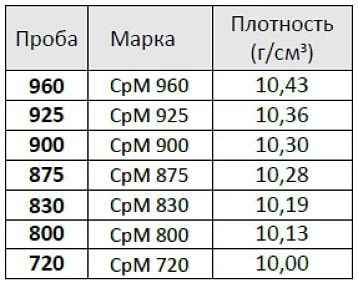
Silver density depending on brand
The metal lends itself well to processing. It can be easily polished, cut, twisted, drawn and rolled. These properties make it suitable for jewelers, but limit the shelf life of the products. Therefore, it is not used in its pure form; copper is added to give the products strength.
925 silver is called sterling silver. Traditional, with the addition of a small amount of copper, almost pure, is considered ideal for making dishes and jewelry. Experiments with the addition of various metals, including silicon, zinc, germanium and even platinum, could not surpass its qualities.
Pure silver is a heavy metal. It has the highest electrical and thermal conductivity of all known metals.
Basic properties:
- the lowest melting point of all precious metals;
- inertness - does not interact with almost any other substances;
- insoluble in acid, except nitric, sulfuric acid and mercury;
- among precious metals it has the greatest ability to undergo chemical reactions.
It does not dissolve in aqua regia, unlike platinum and gold.
An alloy is a combination of pure silver with other metals to improve its certain characteristics. Various alloys are added to silver. Each state has its own documents regulating the amount of impurities, their type and the content of pure metal in each sample. Based on its content in products, the following samples are distinguished:
| Try | Share of silver and copper, % | Density g/(cm^3) |
| Ag | Cu | Rest |
| 800 | 80 | 10.12 |
| 830 | 83 | 10.18 |
| 875 | 87.5 | 10.27 |
| 925 | 92.5 | 10.36 |
| 960 | 96 | 10.43 |
Features of different alloys used in jewelry:
- 960 sterling silver - similar in properties to pure silver. Used for forging and filigree work. It has low mechanical properties and is easily deformed.
- 925 alloy is standard silver. It is characterized by high compliance and stability in operation. Most often used in jewelry.
- 875 alloy - suitable for casting, soldering, bending, forging. The lowest standard that can be used in jewelry. However, it is not popular because it has a yellowish tint.
- 830 test - a hard alloy, used in casting, difficult to machine. Used in industry or in the manufacture of services.
- 800 standard - used for making cutlery, has a yellowish tint and oxidizes quickly. Has the best casting properties.
Silver is a currency metal; bullions are poured from it, which are purchased and sold in a bank at the exchange rate established for today. For production needs, and the metal is used in a wide range of areas, ingots weighing 20 kg are cast. A silver bar is much smaller in volume than a gold bar. There are also round bars with a denomination of 5 US dollars and the highest purity of all - 99.9%.

Silver bars
Alloy of gold and silver
The alloy of gold and silver began to be used in the 3rd millennium BC. This is the time of Ancient Egypt. His people used the precious mixture, for example, for the finials of the pharaohs' pyramids. Their tombs were considered shrines and were inviolable.

By the 20th century, the gold-silver tops of the buildings had disappeared. A couple went to archaeologists, and the rest to black treasure hunters. An alloy of 2 metals is called electron (with emphasis on the first syllable). Let's get acquainted with its formula, origin, features.
History of the electron
The alloy became the first precious one in the history of mankind. There was no need to invent a formula. Gold and silver combine naturally in nature. Near the Nile, in Ancient Lydia, nuggets of precious ore were not in short supply.
In Greek products, silver was 40-54%. This was enough for the alloy to acquire a milky yellow color. For this reason, jewelers of the past called it white gold. The technology for its purification did not yet exist; they did not know how to separate silver.
The alloy of gold and silver is called electron. But the name electrum is also in use. This makes it easier to distinguish the composition from magnesium by ear. The compound of magnesium, zinc and aluminum was created at the beginning of the 20th century. The density of the alloy is low, but the strength is high. The name coincided with the ancient designation of an alloy of silver and gold.

By the way, about the name. “Electron” is translated from ancient Greek as “amber”. Whether the alloy was named after its color, or whether the fossilized resin was named after the metal is unclear. It is only known that the elementary particle electron has been compared to amber. The fact is that the mineral, when in contact with wool or silk, is always charged negatively.
Nuances of the formula and properties of electrum
An alloy of gold and silver is called electrum only if it contains no less than 15 and no more than 50 percent white metal. This is the formula for the natural variety of native gold, which geologists classify as minerals. The electrum stone is officially listed in the gemologist's directory. It occurs in the form of dendrites, that is, crystalline formations.
An alloy of gold and silver with a density of about 14 grams per cubic centimeter and a hardness of approximately 3 points. This is a point, or even one and a half more than pure gold. That is why the ancients installed parts of a mixed composition on the same pyramids.
The white metal made the alloy more resistant to wear and stronger. Electrum was also used in the production of dishes and jewelry. They were harder than ordinary gold and lasted longer. The first Lydian coins in human history were also made of a metal mineral.
The formula of the modern electron has been expanded. They learned to produce the alloy artificially. Therefore, the share of silver in the composition can reach up to 70%. In this case, the color of the mixture completely loses its yellowness. At the same time, the metal retains its radiant shine and the ability to resist oxidation, which is a problem with ordinary silver.
In order to change the properties of the mixture, technologists add base components to it. So, there is an alloy of silver, copper and gold . This is exactly the formula, by the way, for the composition of the 585th sample. True, it does not apply to the electron - only 8% of white metal was used.
Copper is added to modern modifications of the ancient alloy to make it stronger, bringing the hardness to 4-5 points on the Mohs scale. Silver plays the traditional role of making electrum not only harder, but also more malleable. In addition, white metal lowers the melting point, making the work of metallurgists and jewelers easier.
Reserves of natural electrum
The treatises of antiquity indicate that the alloy came to Egypt from Punt. This was the name of the territory in East Africa. Precious bullion was also imported from the Plateau and from a mine east of Rhodesia. Pliny, for example, writes about this.
He also defined that a composition in which less than 15% of silver is considered gold, and an alloy in which more than 70% of white metal is considered silver. Between these “points” the electrum is located.
Electrum is also mentioned in the Odyssey. In the Legend, the concept is used in conversation about gold things coated with silver. However, in modern times these are almost never found. In previous centuries, white metal was sometimes valued higher than gold.

Lands rich in native gold in 3000 BC today are not. The jewelry lying on the surface was collected during times of “fever”. This is one of the reasons for artificially obtaining an electron. There is almost none of it left in nature, especially on an industrial scale.
Small nuggets are found in Western Anatolia. This is the name of one of the regions of Turkey. In addition, natural samples sometimes contain unnecessary impurities, for example, iron and copper. By creating an electron manually, it is possible to create the ideal formula without wasting time on separating tiny amounts of “polluting” substances.
Impurities in silver alloys
Almost every metal in nature has some impurities in its composition. Their presence affects many physical characteristics, for example, density, and, accordingly, the cost of the metal. In silver the following impurities have the following effect:
- Nickel - a nickel content of less than 1% in an alloy increases the density of the alloy, more than 2.5% makes the metal too brittle.
- Lead is considered a harmful alloy, the presence of which is avoided. When the lead concentration is more than 0.05%, it affects silver, making it more brittle when heated.
- Tin - in small quantities, tin reduces the melting point of the alloy; more than 9% makes silver very brittle.
- Aluminum - up to 5% aluminum may contain a pure metal alloy. A larger amount increases the brittleness and brittleness of the alloy.
- Zinc and cadmium are added to silver to make solder. They are used for soldering and repairing silver products - they lower the melting point of the alloy, thereby helping the solder penetrate the pores of the parts being fused. Zinc should contain no more than 20%, otherwise the product will become brittle.
- Silicon - no more than 1.5% dissolves in the metal; a larger amount also makes the alloy weak.
- Carbon is considered a harmful impurity; it makes the metal brittle and does not dissolve in it. They try to completely remove it.
- Phosphorus and sulfur make it dull and brittle. Alloys containing these elements are poorly suited to electroplating.
The process by which silver is purified industrially is called electrorefining. The method involves suspending the metal in an electrolytic bath containing silver nitrate. Silver with impurities is used as the cathode, and pure metal or titanium sheets serve as the anode. The cathode and anode spaces are separated by fiberglass, which retains sludge. Electrolysis is carried out at a voltage of 2-5 volts. The purified metal remains as is on the cathode.
Density indicator
The density of a substance, calculated experimentally under normal conditions, is the ratio of the mass of a substance to its volume. The indicator is measured in g/cm³.
The practical use of this parameter allows you to make arithmetic calculations of the weight and composition of the material. To determine the number of alloy components in a volume, the specific gravity indicator is often used.
Platinum, whose density under normal conditions is 21.09–21.45 g/cm³, is a refractory metal. The melting point is 1773.5°C, the boiling point is 3825°C. The hardness of the metal on the Mohs scale corresponds to 4–4.5.

The density of a substance determines the weight and composition of the metal
To increase the density of the metal, alloys based on it are formed with other metal components:
- birth;
- iridium;
- ruthenium.
Materials with new properties are highly resistant to environmental temperatures and chemical reagents.
Melting alloys
When melted, it easily reacts with oxygen, so it is melted in a covered graphite crucible. The crucible is preheated to remove dirt and moisture. If a ceramic crucible is used, then coal is added to it during melting. Phosphorus or phosphorous copper may be added to prevent oxidation of the alloy.
If a graphite crucible is used, this is not necessary, since oxidation is minor and subsequent cleaning will remove it. The appearance of the surface of the silver when cast can provide vital information - at the right temperature it has a pinkish tint, the surface is clean and shiny. After melting, the top layer, which contains copper oxides, is removed. It is important not to overheat the alloy as this may affect its stability and uniformity.
How to determine a sample by density?
A method for determining the proportion of silver in an alloy was invented by Archimedes. The meaning of this non-destructive testing method is based on weighing the metal in water. In order to determine density, you need to know two quantities - volume and mass.
The weight of the product is determined by weighing on a scale. But how to determine the volume? To do this, we measure the volume of water displaced by the product. Divide mass by volume and get density. Using the table, we determine which metal sample the product belongs to.
Using the same method, you can distinguish other metals from each other. For example, the latest technology in the field of costume jewelry is the production of stainless steel products. To the untrained eye, it can be difficult to determine what kind of white metal a particular piece of jewelry is made of, but this can easily be determined by its density. Steel is much lighter than silver for the same volumes.
Other alloys with gold
In the jewelry industry, alloys are used to make gold jewelry, the main part of which is gold. How are gold alloys different?
- 375 Alloy: This gold is low grade as it is only 37.5% gold, which is only 375 grams of precious metal per kilogram of weight. This alloy can be safely called two-component, since it consists of gold and copper. The latter gives it a reddish tint.
- 500 standard: 50% of this alloy consists of gold. The remaining half comes from impurities. However, this composition of the alloy makes it low-cast, and therefore inapplicable in jewelry.
- 585 standard: products are considered the most popular among jewelry buyers. The structure of such an alloy allows you to “play” with shades. For example, if silver and palladium predominate in 41.5% of the alloy, then in this way you can obtain exquisite white gold, much like platinum. Adding copper and a small amount of silver, taken in equal parts, to the ligature allows you to achieve the effect of rose gold. Another type of 585 alloy is yellow gold. It received its name due to its rich yellow color as a result of the combination of alloy metals in equal parts.
- 750 standard: this metal is considered the most popular in eastern countries, but in Russia products made from it are also popular. Products with a purity of 750 contain at least 75% of the precious metal, while the ligature accounts for only 25%.
- 916 standard: on the Russian jewelry market it is almost impossible to find jewelry made of this metal. At the same time, such an alloy is widely used in jewelry in the East.
- 999 standard: this is pure gold, it can only be found in the form of bank bars.
There are also other gold alloys. As for the medical field, a gold alloy with a purity lower than 750 is never used here. Thus, a metal with a purity of 900, which consists of 900 grams of gold, 60 grams of copper and 40 grams of silver, is used in medicine as a raw material for the production of bridges and crowns Gold with purity 585 is an ideal material for the production of clasps.