Galvanic gilding is the coating of various products made from other materials with a layer of gold using the electroplating method, i.e., by exposure to electric current. Gold plating applied in this way has a thickness of a maximum of tens of microns; The purpose of this technological procedure is to render the product chemically inert to protect it from oxidation and destruction. Gilding also adds luxury to items. Treating new items in this way is called tinting. Galvanization is an economical finishing method that provides high quality coating.
Gold plated jewelry
When they hear the word “jewelry,” many fashionistas imagine unremarkable plastic or metal jewelry that carries no value. High-quality costume jewelry cannot be classified as this type of product; even its base is made of cheap material. Craftsmen usually use base metals for the base of jewelry, and to give it an attractive appearance, they apply a beautiful coating using the galvanic method.
Cupronickel, bronze, brass, pewter or nickel silver are used to produce blanks for jewelry. All these alloys differ from each other in color characteristics and properties. Cupronickel is produced by alloying copper, iron, nickel and manganese. The result is an alloy similar in color to silver. Pewter is made from tin, which makes it easy to cast. Nickel silver is an alloy of zinc, nickel and copper, which, depending on the predominant element, acquires a tint of different shades. Bronze and brass contain copper, which makes their color quite attractive.
Craftsmen achieve the golden color and characteristic “precious” shine using electroplating. The base of the decoration does not have to be metal; it can also be a material of a non-metallic nature. During the galvanic process, the product is coated with a layer of gold or silver. In recent years, the rhodium procedure has often been resorted to.

Electroplating with gold provides the decoration with an attractive appearance. Reviews of such products are divided into two parts. Lovers of good jewelry speak positively, arguing that gold-plated jewelry has the effect of a precious metal, but for less money. High-quality coating and painstaking work of an experienced craftsman can bring the most daring design ideas to life. Others have a negative attitude towards products of this kind, since they only recognize precious jewelry.
Methods of applying gold plating
Among the possible options for applying gilding, experts distinguish two methods: mechanical and electrochemical. The mechanical method involves covering the surface with gold leaf. Gilding with the thinnest sheets of metal has been used since ancient times; over many centuries, the essence of the procedure has remained virtually unchanged. Gold leaf plating can be oil-based or glue-based; in the first case, the metal is glued to oil varnish, and in the second, to polyment. The oil-based coating turns out matte; to achieve the effect of a shiny surface, experts resort to using an adhesive base. Work on creating an adhesive coating can be carried out exclusively indoors, since such gilding is characterized by high sensitivity to moisture.

The electrochemical version of surface treatment with precious metal is a galvanic method of applying gold to a product. What it is? During the work, the master applies, using electric current, a thin layer of yellow precious metal, the size of which can reach fractions of microns.
Like any coating, electroplating has its advantages and disadvantages over other options. There are quite a few advantages of coating obtained in this way. These include a high level of wear resistance, excellent reflectivity, high current conductivity, the ability to protect the product from aggressive external factors, the effects of corrosive and oxidative processes. When applying galvanic coating, the specialist carrying out the work can control the thickness of the precious metal layer. Due to the listed properties, galvanic gilding is widely used both in surface decoration and for creating technical parts of devices.

Electrochemical deposition of gold on the surface.
For a long time, the main disadvantage of the method was considered to be the limited scope of its application. The electrochemical method of applying gold to a surface assumes that this surface is conductive, that is, made of metal. Thanks to scientific advances, this problem has now been partially resolved: special technologies make it possible to gild dielectric materials using conductive varnishes and films.
Golden varnish for brass (passivation of brass)
When brass is passivated, a stable protective film similar to gold plating is formed. This film is not afraid of moisture, so fishermen passivate brass lures.
The cleaned, polished and degreased part is dipped for 1 second in a solution prepared from 1 part nitric and 1 part sulfuric acid, and immediately transferred to a strong solution of potassium dichromate (chrompic) for 10-15 minutes. After this, the part is washed and dried.
Literature: V. G. Bastanov. 300 practical tips, 1986
Gilding technology
Gold plating is a process of depositing a metal film. The thickness of the film can be different; depending on the purpose of gilding, a specialist can apply a layer on the product with a thickness ranging from a fraction of a micron to a fraction of a millimeter. The whole process is divided into three stages: first the surface must be prepared, then a layer of metal is applied and final processing is carried out.

A high-quality coating will only be achieved if the surface to be treated is well prepared. The product is pre-polished mechanically. To do this, use sandpaper, special pastes or grinding machines. Then the surface is degreased in an organic solvent. Alcohol, acetone or gasoline can cope with this task. Another mandatory procedure before galvanic treatment is pickling - removing existing contaminants, oxides and rust from the surface.
Sometimes it is necessary to electroplate only part of a part; for this, the remaining areas of the product must be protected from the effects of electrolyte and gold deposition. To do this, before applying the precious coating, acid-resistant varnish is applied to areas that are not subject to gilding.
The part is plated with gold using galvanic baths. The work is carried out using conductive suspensions and drums made of acid-resistant materials or in bell installations that ensure excellent electrical contact. The galvanic bath must also have an acid-resistant coating so as not to be destroyed under the influence of the electrolyte and equipment in the form of steam jackets. The entire process takes place at high temperature and the required current density, which are supported by automatic regulators. It is almost impossible to carry out galvanic procedures at home, since this requires not only special equipment, but also rare chemical reagents.

Upon completion of the work, the product is coated with a thin metal layer, which provides the best characteristics for the part. For decorative products, it is important to acquire an attractive appearance and the desired shade, for industrial parts - the ability to resist corrosion, improve electrical contact and facilitate the soldering process. Sometimes galvanic coating is used to increase the volume of a product. Depending on what properties need to be imparted to the part, electroplating can be done with both gold and other elements: silver, chromium, nickel.
Electrolytes for gilding metal surfaces.
2.1 Cyanide electrolytes.
When gilding, cyanide and non-cyanide electrolytes are used. Cyanide compounds, in turn, are divided into acidic, alkaline and neutral. All cyanide electrolytes contain some amount of free potassium cyanide.
In alkaline and neutral solutions, gold is found mainly in the form of the K[Au(CN)2] complex. The presence of the trivalent complex K[Au(CN)4] is possible in small quantities.
2.1.1 Cathode process in cyanide gold plating electrolytes.
The reaction that determines the electrode potential:
[Au(CN)2]- = AuCN + (CN)- AuCN = Au+ + (CN)-
Cathode polarization curves for an alkaline electrolyte are shown in Figure 1; the polarization of gold electrodeposition from acidic and neutral solutions is in the region of more positive values.
Figure 1 - Polarization curves of gold in alkaline cyanide electrolyte (g/l Ag in terms of metal): 1 - 17, 2 - 30.
Let's look at the polarization curves in Figure 1 in more detail:
• In sections 1 and 2 of the curves at a potential from -0.4 to -0.6 V, AuCN or Au2(CN)2 participate in the elementary reaction. • In the 3rd and 4th sections at a potential from -0.7 to -0.9 V - [Au(CN)2]-. • In section 5 - [Au(CN)2]- and H+. Under the influence of a strong electric field in the DES at the cathode, the adsorption of the [Au(CN)2]- anion occurs with the positive end towards the cathode, which facilitates the discharge process:
[Au(CN)2]- + e = [Au(CN)2]2- [Au(CN)2]2- = Au + 2(CN)-
The trivalent gold complex has been studied very little. One of the researchers was A. Knodler. It is known that heating an alkaline electrolyte and increasing the pH promotes the formation of monovalent gold. If we consider the reactions:
[Au(CN)4]- = [Au(CN)2]- + (CN)2 (CN)2 + 2(OH)- = (CN)- + (CNO)- + H2O [Au(CN)4 ]- + 2(OH)- = [Au(CN)2]- + (CN)- + (CNO)- + H2O
then it becomes obvious that the decomposition of the trivalent gold complex should indeed accelerate with increasing pH (increasing OH- concentration).
In neutral and acidic solutions, the trivalent complex is stable even when heated.
If a trivalent gold complex is present in the solution, its decomposition is facilitated by metallic gold and free cyanide:
[Au(CN)4]- + 2Au + 2(CN)- = 3 [Au(CN)2]-
Metallic gold can be anodes, gold-plated parts left without current, particles of gold sludge and coating residues that have fallen into the bath. The cathodic current efficiency in alkaline solutions can reach 70-80%, neutral - almost 100%, acidic - 30-40%. Operation at low current densities is accompanied by a decrease in VT. The already deposited gold coating dissolves according to the following reactions:
2Au + 4KCN + O2 + 2H2O = 2K[Au(CN)2] + H2O2 + 2KOH 2Au + 4KCN + H2O2 = 2K[Au(CN)2] + 2KOH
2.1.2 Anodic process in cyanide gold plating electrolytes.
The anodic process during gold plating has some anomalies - both in the mechanism and in the current output. The anomalies are mainly associated with the possibility of the formation of trivalent gold complexes.
It is possible to use soluble and insoluble anodes:
• Soluble anodes for gold plating can only be used in alkaline cyanide solutions. They can dissolve chemically in cyanide, which increases the anodic current efficiency. Over time, the trivalent complex is restored and the anodic process stabilizes.
• Platinized/irridified titanium plates or meshes are used as insoluble anodes.
Gold alloy plating
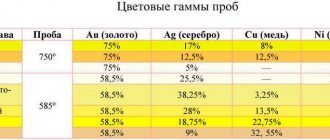
To coat products, gold alloys are most often used, to which a ligating component is added. An additional element in the alloy allows you to give the part the necessary qualities and the desired shade. In Russia, preference is given to gold with a reddish tint; in the USA and some other countries, priority is given to coatings of lemon yellow or brass shades.
In order for electroplating to have a tint not like gold, but like platinum, the working alloy must consist of gold and nickel, the percentage of which must be at least 8-10%. This coating will have a white tint and will also be highly hard compared to a pure gold layer. The higher the proportion of nickel in the alloy, the higher the hardness and wear resistance of the final surface will be. Electroplating based on gold and nickel is used abroad for jewelry. Due to their high resistance to corrosion processes, they can also be used for technical purposes.
An alloy of gold and copper is used in Russia to coat elements of wristwatches. The progress of the process depends on the concentration of free cyanide in the electrolyte: the higher the concentration of the substance in the electrolyte, the lower the copper content in the resulting coating. When the process is carried out under conditions of neutral electrolytes, a copper-gold coating with a thickness of 20 microns can be obtained.
In addition to the listed alloys, gold-silver and gold-antimony compositions are also used. The percentage of elements in the final coating depends on the characteristics of the electrolyte and the chemical reagents used.

Gold-antimony electroplating has earned positive reviews for its use in gilding eyeglass frames. A coating of this composition is characterized not only by increased wear resistance, but also by an attractive appearance. Depending on the thickness, it can turn out semi-shiny or shiny. Such properties, together with a high level of resistance to mechanical stress, allow the alloy to be used for decorative purposes.