Industrial method
When extracting gold from so-called gold-bearing sands, you have to work with a suspension of approximately equally small particles of gold and grains of sand, which need to be separated from each other. You can do this by washing, or you can use sodium or potassium cyanide - there is no difference. The fact is that gold forms a soluble complex with cyanide ions, but sand does not dissolve and remains as is.
The key point in this reaction is the presence of oxygen (there is enough of it in the air): oxygen oxidizes gold in the presence of cyanide ions and a complex is obtained. If there is insufficient air or without cyanide, the reaction does not proceed.
Now this is the most common method of industrial gold production. Of course, there are still many stages before obtaining the final product, but we are specifically interested in this stage: cyanide solutions - what gold dissolves in.
Chemical method
The chemical method of extracting gold from radio components is the most common. All you need to implement it is nitric acid. The fact is that this acid dissolves almost all metals except gold. Upon completion of the process, you will only need to collect the resulting gold foil.
At the end of the reaction, it will be possible to observe the release of steam from the acid. This suggests that nitric acid has not yet lost its properties. The container into which the nitric acid will be poured must be made of aluminum, since it also does not react with nitric acid. To speed up the gold mining process, the acid can be heated. But you need to know that under no circumstances should it be heated above 60 degrees.
The rate of reaction also depends on the amount of acid. It is recommended that the volume of acid be 3 or more times greater than the volume of the radio components being cleaned. It is also desirable that the pan in which the entire process will take place has high sides, since bubbles will form during the reaction. During the reaction, the acid will decompose into nitrates, which will increase its volume - this also needs to be taken into account.
Before adding a gold-plated radio component to the acid, it needs to be prepared. Namely, you need to remove all unnecessary elements from it, if possible, leaving only the part with gold. If this is not done, the effectiveness of the solution will decrease and it will have to be periodically replaced with a new one.
Sell gold
The chemical method also includes the extraction of gold using aqua regia. Such a solution is made from nitric and hydrochloric acids, when mixed, a strong solvent is obtained that can dissolve any metal. At the end of the reaction, hydrazine is added to the container, without which it will not be possible to detect gold particles. After adding hydrazine, a brown mass is formed, which is gold. However, such gold has a very low purity. To obtain gold of a higher standard, the etching process must be repeated 3-4 more times.
Amalgam
The amalgamation process is also used in industry, only when working with ores and hard rocks. Its essence lies in the ability of mercury to form an amalgam - an intermetallic compound. Strictly speaking, mercury does not dissolve gold in this process: it remains in solid form in the amalgam.
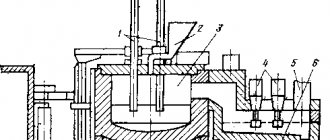
During amalgamation, the rock is wetted with liquid mercury. However, the process of “pulling” gold into amalgam is long, dangerous (mercury vapor is poisonous) and ineffective, so this method is rarely used anywhere.
Aqua regia
There are many acids that can corrode living tissue and leave terrible chemical burns (even death). However, there is no single acid in which gold dissolves. Of all the acids, only the famous mixture - aqua regia - can affect it. These are nitric and hydrochloric (hydrochloric) acids, taken in a ratio of 3 to 1 by volume. The remarkable properties of this hellish cocktail are due to the fact that acids are taken in very high concentrations, which greatly increases their oxidizing ability.
Aqua regia begins to act when nitric acid begins to oxidize hydrochloric acid first, and during this reaction, atomic chlorine is formed - a very reactive particle. It is she who attacks gold and forms a complex with it - chlorauric acid.

This is a very useful reagent. Very often, gold is stored in the laboratory in the form of crystalline hydrate of such an acid. For us, it only serves as confirmation that gold dissolves in aqua regia.

It is worth paying attention once again to the fact that it is not one of the two acids that oxidizes the metal in this reaction, but the product of their mutual reaction. So if you take, for example, “nitrogen” alone - a well-known oxidizing acid - nothing will come of it. Neither concentration nor temperature can make gold dissolve in nitric acid.
How to dissolve gold jewelry step by step?
First of all, if the material is scrap precious metal or a product with impurities, cleaning should be carried out using ferromagnetic intervention.

The process then continues in the form of treatment with pure nitric or other acids. Only after this can you begin direct dissolution.
You should start with accurate measurements of proportions. That is, if the mass of the potential material is 1 gram, you should prepare 3.75 milliliters of concentrated hydrochloric acid, then mix everything in a container.
Almost immediately after mixing, dissolving processes should occur. As soon as the reaction is complete, the solution is drained and repeated as in the first case.
After adding 2 portions of hydrochloric acid, the flask must be heated. During the heating process, the next component in the form of nitric acid is slowly introduced.
If the initial ratio of acids is 3 to 1, then per 1 gram of metal you will need 1.25 ml of nitric acid and in no case more, since it will be difficult to isolate in the next stage.
Within a short time, the precious metal will completely dissolve, but it should be noted that the definition of completely means exclusively the gold component. Other components, for example, silver, tend to be passivated.
Once the entire process has reached the completion stage, you should wait 20-30 minutes, maintaining the heating temperature at the same level.
One small but important note! It is not enough to know what gold is dissolved in; you must follow clear recommendations and be sure to observe safety precautions.
Under no circumstances should the heating process be allowed to boil. If this happens, the precious metal will begin to precipitate ahead of time, and this will significantly complicate the further stage of deposition of the aurum in its pure form.
You may be interested in: How to find out the gold rate at Sberbank of Russia?
Bleaching
Unlike acids, particularly hydrochloric acid, individual substances can become what gold dissolves in. A widely known household bleach is a solution of chlorine gas in water. Of course, you can’t do anything with an ordinary store-bought solution; higher concentrations are needed.
Chlorine water acts as follows: chlorine dissociates into hydrochloric and hypochlorous acids. Hypochlorous acid decomposes under light into oxygen and hydrochloric acid. In such decomposition, atomic oxygen is released: like atomic chlorine in the reaction with aqua regia, it is very active and oxidizes gold for the sweet soul. As a result, a complex of gold and chlorine is again obtained, as in the previous method.

Acids used in jewelry making
Nitric acid (HN03) is a colorless liquid that fumes slightly in air. Density 1.5; boiling point °3.8 °C. At a temperature of -42 °C it hardens into a transparent crystalline mass. Mixes with water in any ratio. '1°D decomposes under the influence of light into water, oxygen and nitrogen dioxide. Nitric acid is one of the most powerful acids. It acts on almost all metals (with the exception of gold, platinum and some rare metals), turning them into nitrate salts. Used for the preparation of assay reagents for the determination of impurities of precious metals (except silver).
Sulfuric acid (H2S04) is a colorless oily liquid. Density 1.84; boiling point 338 °C. At a temperature of 10.4 °C it forms a solid crystalline mass. Dissolving in water, it releases a large amount of heat. To avoid burns when mixing acid with water, pour the acid into the water, and not vice versa. Concentrated sulfuric acid, when heated, dissolves almost all metals except platinum, gold and some platinum group metals. It is used for extracting gold from ores and for preparing bleaching solutions.
Hydrochloric acid (HO) is hydrogen chloride dissolved in water, a colorless liquid with a pungent odor. It smokes slightly in the air. The density of ordinary concentrated acid is 1.19; it contains 37% HC1. The acid used for technical purposes is usually yellow and contains 27.5% HC1. Hydrochloric acid is highly soluble in water and easily reacts with many metals, forming salts and releasing hydrogen. It is used for the preparation of bleach and assay reagents.
Aqua regia is a mixture of hydrochloric and nitric acids in a ratio of 2:1 and 3:1, reddish-brown in color. Dissolves all metals except rhodium, iridium, osmium. Platinum dissolves only in hot aqua regia. Used in the preparation of assay reagents.
Orthophosphoric acid (H3P04) - colorless crystals. Density 1.8; melting point 42.35 °C. Very soluble in water. Considered non-poisonous. When heated to 215 °C, it transforms into pyrophosphoric (H4P2O7) acid. Phosphoric acid is used to prepare electrolytes for rhodium plating jewelry.
Boric acid (H3B03) is a white crystalline substance and belongs to the group of very weak acids. Density 1.4... 1.5. It dissolves easily in hot water, but crystallizes when it cools, since it is slightly soluble in cold water. When heated, boric acid loses water, turning into meta-boric acid (HB02), then tetraboric acid (H2B407) and, finally, into boric anhydride (B2O3). It is used as a flux when melting and soldering precious metals, and also as a component of fluxes.
Other halogens
In addition to chlorine, other elements of the seventh group of the periodic table also oxidize gold well. It is difficult to fully say about them: “that in which gold dissolves.”
Gold can react with fluorine in different ways: during direct synthesis (at a temperature of 300-400°C), gold fluoride III is formed, which immediately hydrolyzes in water. It is so unstable that it decomposes even when exposed to hydrofluoric acid, although it should be comfortable among fluoride ions.
Also, by the action of the strongest oxidizing agents: fluorides of noble gases (krypton, xenon), gold fluoride V can be obtained. Such fluoride generally explodes upon contact with water.
With bromine, things are somewhat simpler. Bromine is a liquid under normal conditions, and gold dissipates well in its solutions, forming soluble gold bromide III.
Gold also reacts with iodine when heated (up to 400°C), forming gold iodide I (this degree of oxidation is explained by the lower activity of iodine compared to other halogens).
Thus, gold certainly reacts with halogens, but whether gold dissolves in them is controversial.
Gold (Au)
- Designation - Au (Aurum);
- Period - VI;
- Group - 11 (Ib);
- Atomic mass - 196.96655;
- Atomic number - 79;
- Atomic radius = 144 pm;
- Covalent radius = 134 pm;
- Electron distribution - 1s22s22p63s23p63d104s24p64d104f145s25p65d106s1;
- melting temperature = 1064.18°C;
- boiling point = 2856°C;
- Electronegativity (according to Pauling/according to Alpred and Rochow) = 2.54/1.42;
- Oxidation state: +5, +3, +1, -1;
- Density (no.) = 19.3 g/cm3;
- Molar volume = 10.2 cm3/mol.
Gold is one of the most “unusual” metals known to man for a long time - our ancestors used gold mostly for cultural and religious purposes, and also as one of the most reliable means of payment.
Gold occurs in nature in the vast majority of cases in the form of nuggets. Gold can form natural solid solutions with some metals:
- electrum - silvery gold;
- aurocuprid - coppery gold;
- platinum gold.
In the form of compounds, gold is much less common in nature (calagerite AuTe2, aurostibite AuSb2). Quite a lot of gold is contained in sea water, but mining such gold is unprofitable.
Gold in D.I. Mendeleev’s Periodic Table of Chemical Elements, numbered “79”, belongs to the transition metals (See Atoms of transition elements).
Rice. Structure of the gold atom.
The electronic configuration of a gold atom is 1s22s22p63s23p63d104s24p64d104f145s25p65d106s1 (see Electronic structure of atoms). Gold is one of the most inactive chemical elements - it does not oxidize in air even in the presence of moisture, and does not interact directly with oxygen, hydrogen, nitrogen, carbon, or phosphorus.
Physical properties of gold:
- golden yellow metal;
- very plastic and soft - you can roll it into transparent foil several microns thick (gold leaf), which can be used to cover a variety of artistic and religious items (gold plating), giving them an aesthetically attractive appearance that lasts for a long time;
- has good electrical and thermal conductivity.
Chemical properties of gold:
- reacts with halogens when heated: 2Au + 3Cl2 = 2AuCl3;
- does not dissolve in alkaline and acidic solutions;
- dissolves in mixtures of acids: HCl+HNO3 (regia vodka) and H2SO4+HNO3: Au + 3HNO3 + 3HCl = AuCl3 + 3NO2 + 3H2O;
- metallic gold goes into solution in the form of a complex salt in aqueous solutions of potassium and sodium cyanide in the presence of oxidizing agents: 4Au + 8KCN + 2H2O + O2 = 4K[Au(CN)2] + 4KOH;
- easily dissolves in mercury to form an alloy (amalgam).
When slightly heated, oxygen compounds of gold easily decompose explosively; for example, gold fulminate explodes at 145°C.
Gold compounds are quite easily hydrolyzed and reduced to free metal in aqueous solutions, since they are unstable compounds: when heated, gold (III) hydroxide dehydrates to form gold (III) oxide, which, in turn, decomposes to form free gold at 160°C: 4Au(OH)3 → 2Au2O3 → 4Au + 3O2.
Gold(III) hydroxide and oxide are amphoteric compounds that react with acids and bases to form complex compounds:
- Au(OH)3 + KOH = K[Au(OH)4]
- Au(OH)3 + 4HCl = H[AuCl4] + 3H2O
Application of gold:
- in jewelry;
- as an international means of payment;
- in electrical engineering for gilding contacts;
- for coating metal surfaces;
- in medicine for the manufacture of instruments and prostheses;
- as a catalyst for certain reactions in the chemical industry;
- The radioactive isotope of gold is used in oncology to treat tumors.
Top of page
Lugol's solution
In fact, iodine (common iodine I2) is insoluble in water. Let’s dissolve its complex with potassium iodide. This compound is called Lugol's solution - and it can dissolve gold. By the way, it is often used to lubricate the throat of those with a sore throat, so not everything is so simple.
This reaction also occurs through the formation of complexes. Gold forms complex anions with iodine. It is used, as a rule, for etching gold - a process in which interaction occurs only with the surface of the metal. Lugol's solution is convenient in this case because, unlike aqua regia and cyanide, the reaction is noticeably slower (and the reagents are more accessible).
Bonus
When we said that single acids are something in which gold does not dissolve, we lied a little - in fact, such acids exist.
Perchloric acid is one of the strongest acids. Its oxidizing properties are extremely high. In a dilute solution they show up poorly, but in high concentrations they work wonders. The reaction produces its salt, gold perchlorate, which is yellow and unstable.
Of the acids in which gold dissolves, there is also hot concentrated selenic acid. As a result, a salt is also formed - gold selenate of red-yellow color.