Platinum is the Cinderella of the metal world. In its original state, it looked like grimy silver, which came across to the Spaniards who arrived in the New World with Columbus to get rich. The Columbia gold mines also contained “dirty silver” that miners discarded. The dirty-looking grains were difficult to separate from the gold, they melted with difficulty, and it was not easy to separate them from the gold...
Miners who called silver "plata" called the dirty gray pieces of metal "platina". Then the noble metal was “frog, rotten silver”, just silver.
The story about counterfeiters and Ural hunters
The cheap “silver” gave the counterfeiters a great idea. The annoying metal was added to gold: the color of the alloy did not change, and the fakes were of high quality.
The Spanish crown, having learned about the tricks of the scammers, ordered the “harmful metal” to be drowned. Naturally drown in the waters of the Atlantic - in Colombia and Spain. Later, the Spanish court decided to make some money on the “rotten silver” and add it to coins.
The sad story of the “metal Cinderella” ended by the 19th century.
Scientists became interested in platinum and discovered that it has a “family.” Together with the “head” there are five family members. These are rhodium, iridium, ruthenium, palladium, osmium. The classification was carried out by scientists from different countries.
Interesting: Ural hunters, encountering platinum placers, used metal grains instead of shot. It was the most expensive shot in the world.
Physical and chemical properties
Platinum is a noble metal, shiny, malleable, silvery-white in color.
Platinum crystals
Chemical properties of metal.
| Property | Indicators |
| Oxidation states | 0, 2, 4 |
| Covalent radius | 130 picometers |
| Electronegativity | 2.28 on the Pauling scale |
Physical properties:
- Mohs hardness 3.5;
- structure (crystal lattice) face-centered;
- density 21.1-21.45 g/cm3;
- melting point 1768.3 °C.
| Properties of the atom | |
| Name, symbol, number | Platinum (Pt), 78 |
| Atomic mass (molar mass) | 195.084(9)[1] a. e.m. (g/mol) |
| Electronic configuration | [Xe] 4f14 5d9 6s1 |
| Atomic radius | 139 pm |
| Chemical properties | |
| Covalent radius | 130 pm |
| Ion radius | (+4e) 65 (+2e) 80 pm |
| Electronegativity | 2.28 (Pauling scale) |
| Electrode potential | Pt←Pt2+ 1.20 V |
| Oxidation states | 4, 2, 0 |
| Ionization energy (first electron) | 868.1 (9.00) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 21.09-21.45[2][3] g/cm³ |
| Melting temperature | 2041.4 K (1768.3 °C, 3214.9 °F)[2] |
| Boiling temperature | 4098 K (3825 °C, 6917 °F)[2] |
| Ud. heat of fusion | 21.76 kJ/mol |
| Ud. heat of vaporization | ~470 kJ/mol |
| Molar heat capacity | 25.85[3] J/(K mol) |
| Molar volume | 9.10 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 3.920 Å |
| Debye temperature | 230.00 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 71.6 W/(mK) |
| Thermal expansion | (25 °C) 8.8 |
| Young's modulus | 168 GPa |
| Shear modulus | 61 GPa |
| Volume control module | 230 GPa |
| Poisson's ratio | 0,38 |
| Mohs hardness | 3,5 |
| Vickers hardness | 549 MPa |
| Brinell hardness | 392 MPa |
| CAS number | 7440-06-4 |
We recommend: BISMUT - radioactive and safe
This precious metal is practically resistant to rust. Under normal conditions, it reacts slowly with aqua regia; when heated - give it time - it will dissolve in sulfuric acid and liquid bromine. Other acids (inorganic, organic) have no effect on platinum.

Now platinum is appreciated; it is called the “queen of metals.”
How platinum raw materials are processed
The metal is mechanically cleaned of foreign substances. Then comes the stage of chemical cleaning, which is aimed at improving quality and increasing the properties of the metal by chemically removing impurities.
At this stage of cleaning, the nuggets are heated in large porcelain containers, into which aqua regia is added. This process separates base metals that dissolve. But an insoluble precipitate containing other elements is also released.
The sediments are filtered and then re-treated with aqua regia. The osmium and iridium obtained during this process are further used in industry and are therefore extracted from the boilers.
As a result of these actions, platinum remains in two complexes:
An element called HCl is introduced into the second complex. It is he who transfers (NO) 2[PtCl6] to the first complex. This results in a single complex, which includes palladium, osmium and iridium, which are classified as noble metals. They must be converted into compounds (Ir3+, Pd2+), which cannot be affected by ammonium chloride, so that the elements do not precipitate. Next, the solution is changed by heating with sulfuric and oxalic acids.
After ammonium chlorine is introduced, platinum precipitates in the form of gold-colored granules. The platinum remains in solution. The extracted metal is then cleaned with ammonia and dried.

Only after such a long purification process is platinum ready for smelting.
At what temperature does it melt?
The melting point of platinum is considered to be 1773.5 °C, however, there are recommendations for melting certain types:
| Alloys | Melting temperature | Plastic | Specific gravity | Strength |
| Pt850/Ir | 1800—1820 °C | 15% | 21,5 | 160 HV |
| Pt900/Ir | 1780—1800 °C | 20% | 21,5 | 110 HV |
| Pt950/Ir | 1780—1790 °C | 30% | 21,45 | 80 HV |
| Pt 900/ Co30/ Pd70 | 1730°C – 1740°C | 22% | 20,4 | 120 HV |
| Pt850/Pd | 1730—1750 °C | 76 HV | ||
| Pt900/ Pd | 1740—1755 °C | 22% | 19,8 | 72 HV |
| Pt950/Pd | 1755—1765 °C | 22% | 19,8 | 68 HV |
| Pt950/Co | 1780—1765 °C | 20% | 20,8 | 135 HV |
| Pt960/Cu | 1725—1745 °C | 29% | 20,0 | 108 HV |
| Pt950/ Co/ Cu | 1750—1760 °C | 22% | 20,1 | 120 HV |
| Pt950/ 15In/ 30Ga | 1550—1625 °C | 26% | 20,22 | 200 HV |
| Pt950/ Ru | 1780—1795 °C | 34% | 20,7 |
Earth and star maternity hospital platinum
The main platinum deposits are located in the countries:
- Zimbabwe;
- USA;
- SOUTH AFRICA;
- China;
- Russia.
This is approximately 90% of the world reserve (according to some analysts). Others give the same 90% of platinum in nature to one South African deposits. Such strange “analytics” show that the bowels of the Earth have been explored approximately.

There are small deposits in South America, Canada and a number of countries.
We mine platinum
About interesting and important mining sites of the “queen of metals”. Platinum is found in placers and ultramafic bedrock.
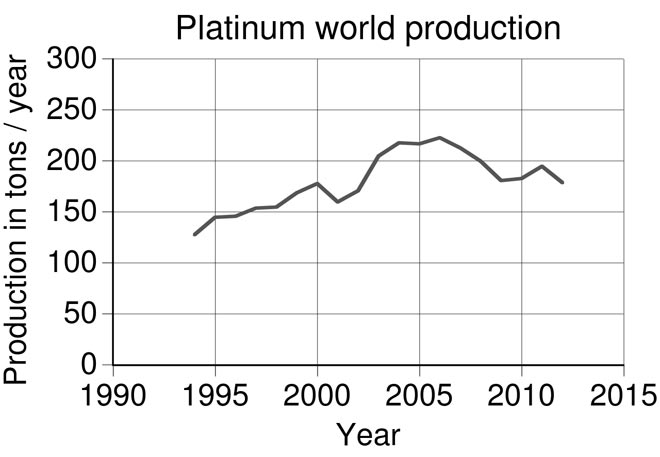
World platinum production (tonnes per year) over recent decades.
The process of formation of the noble metal occurred not only on Earth. Some of the asteroids that fell to Earth were filled with platinum group metals.
South Africa
The largest “basket” on earth with platinum and platinum group metals is located in South Africa. This is the Bushveld complex. It looks like a saucer with a diameter of about 370 km. Horizons (reefs), lenses, and platinum veins stretch from the edges of the saucer to the center. Their thickness ranges from 1 to 90 meters; some reefs are mined by open-pit mining.
¾ of all platinum is mined here.
Russia
We had the richest placer deposits in the Urals. Since 1828, the country has minted platinum coins in denominations of 3, 6 and 12 rubles. For coins, native metal was used, not purified from accompanying metals. The coins were minted for only 17 years, then they began to be withdrawn from circulation.
After 100 years, the Ural deposits were depleted.
Currently, silver mining is carried out in Taimyr, on the Kola Peninsula, in the Khabarovsk and Krasnoyarsk territories.
Canada
In central Ontario, nickel and palladium are mined, and platinum is a “prize” for miners. By the way, most likely, the platinum group metals here are of extraterrestrial origin. Once upon a time a huge meteorite fell here.
The Lac des Iles mine in western Ontario uses open-pit mining for platinum group metals. Recently, an underground mine was opened here, where the content of noble platinoids is higher.
USA
There are only two mines here, both in Montana. However, the ore is rich, the content of platinum group metals is about 20 grams per ton of ore.
In 2015, a small asteroid (less than a kilometer in diameter) flew close to Earth. It differs from the mass of other celestial bodies in its “filling”. The core of the celestial stone, as astronomers have found, consists of platinum, gold and other attractive metals. It's a shame we didn't catch it...
We recommend: IRIDIUM - a gift from space
Platinum for man
The scope of application of the precious metal includes the oil refining industry. Here you cannot do without catalysts containing platinum. The lion's share goes here (about half of the precious metal mined). The chemical industry uses the noble metal in the production of nitric acid.
Platinum is indispensable in radio engineering, electrical engineering, telemechanics, and precision instrument making.
“Silver” is used in medicine. Here, surgical instruments are made from platinum alloys. There are methods for treating cancer using cis-isomers, derivatives of divalent platinum.
The activity of the Green Party has led to an increase in consumption of the “queen of metals” in the automotive industry. Tightening standards for harmful emissions into the atmosphere has led to the equipping of cars with autocatalysts.
The Russian kilogram standard is made from an alloy of platinum and iridium.
Informative: the police and casinos use platinum-coated mirrors. From the illuminated side it works like an ordinary mirror, from the shadow side it is transparent, like glass.
Melting platinum at home
If a situation arises when you need to melt platinum at home, you first need to familiarize yourself with this process. Before the procedure, prepare the necessary tools and materials. Platinum can be melted using homemade gas burners using oxygen cylinders. A welding machine is also used for these purposes. A porcelain crucible is used as a container.

Laboratory porcelain crucible
The mixture is prepared in the same way as in industrial conditions. The melting process itself is performed as follows.
- The platinum alloy is placed in a crucible and heated with a gas burner or other homemade devices until the required temperature is reached.
- The type of metal cast depends on the mold created and personal preference.
- After melting, the platinum must cool completely.
- The finished product is removed from the mold using tools and taking the necessary precautions.
As you can see, you can melt platinum yourself, although the final result largely depends on the skills of the master, the quality of materials and knowledge of technology. It is almost impossible to obtain a pure product without impurities without special equipment, because... All melting methods are not ideal. If you need pure metal, it is better to contact specialists.
Platinum jewelry
Platinum jewelry is not very popular in Russia. Most likely, it’s a matter of habit, the belief that “if you want to look rich, wear gold.” In addition, the price of this noble metal “bites”. 1 gram costs from 5,500 to 7,500 rubles (the price largely depends on the seller’s appetites).

The fashion for jewelry comes to Russia from the West, and the fashion for platinum products has reached us. Cartier has been working with precious metal for a long time. Inserts in platinum earrings, rings, and necklaces are often made of diamonds. The spring transparency and cold fire of the stone are amazingly suited to the dispassionate purity and whiteness of platinum. Colorless or white stones look good when framed in elegant metal.
- The advantages of a platinum frame are corrosion resistance, hardness, beauty, expensive and respectable appearance.
- drawback , but a significant one. Platinum is far from being a democratic metal; not everyone can buy jewelry made of the precious metal.
Platinum alloys for jewelry
Alloys of platinum with various metals are used in jewelry. These are cobalt, copper, tungsten, iridium. They add useful properties to the alloy: improve mechanical properties, extend the service life of products, and impart softness.
The following alloy samples are accepted in Russia:
- 950 - the most popular among jewelers and consumers;
- 900 - used less frequently than 950; it has a weaker white color and less shine;
- 850 - not often used in jewelry production: the shine and whiteness of platinum is “killed” by silver.
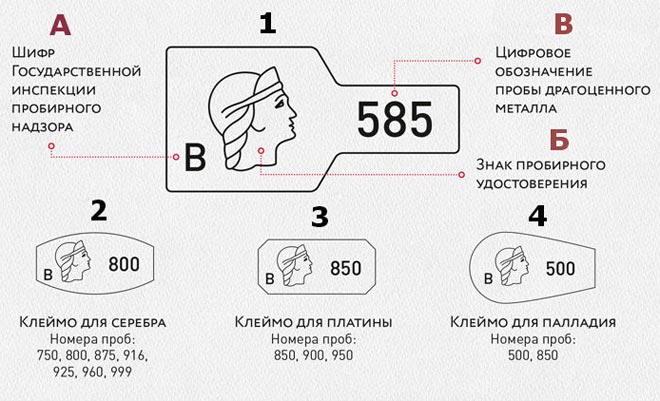
How to replace platinum
You don’t have enough money to buy platinum jewelry, but you really want it - buy white gold jewelry. Sometimes these metals are confused.
The main difference between platinum and white gold is that there is no platinum in white gold. Technically, white gold is a jewelry alloy of gold with silver, nickel or palladium.
We recommend: TIN - a diverse metal
Recent studies show that more than 10% of people suffer from nickel allergies. In Europe, the use of nickel in jewelry is prohibited by law.
Informative: mankind has been using an alloy of gold and silver for a very long time. The alloy was called electrum, electrum, and was mentioned by the ancient authors Pliny, Homer, and Archimedes. The first coins in human history were made of electrum. It happened in Lydia, a very long time ago, in the 7th century BC.
Is it possible to melt platinum at home?
I wonder if it is possible to melt platinum at home - I’ll immediately make a reservation that you should start such actions if you understand the topic! Self-smelting methods are artisanal and sometimes unsafe.
It is almost impossible to obtain a pure product without impurities under such conditions; all melting methods are not ideal. It is better to seek help from a specialist.
Necessary equipment and materials
Some amateurs melt platinum using self-assembled gas burners using oxygen cylinders, while others do it with a welding machine. A porcelain crucible (heating pot) is also required.
Batch preparation
In general, the preparation process is no different from the industrial one, of course, within the limits of your capabilities, which are extremely limited at home.

Melting process
The alloy is placed in a crucible and heated with a gas torch, welding inverter or other homemade devices until the required temperature is reached.

Receiving castings
Cast metal comes in different forms depending on the mold created. Your preferences come into play here. The main thing is to let the platinum cool and use precautionary tools.

Caring for platinum jewelry
Jewelry care includes:
- cleaning by a professional (especially if the jewelry contains precious stones);
- checking for good fixation of stones in the product;
- unlike you, jewelry does not benefit from cosmetics and household chemicals;
- inspect the jewelry for cracks, scratches, or chips. If you find damage, run to the jeweler.
Store jewelry in separate cases, boxes, bags. This way, products made from different metals and stones (with different hardnesses) will not scratch one another.

Platinum image of Lenin on the Order of Lenin.
Wash earrings and rings with a neutral soap solution using a soft brush. Place the chains in a glass, fill with soapy water and swirl the contents.
Then rinse the product under running water and dry.