Greetings, inquisitive friends of nature, who among us is not familiar with such a noble gift of nature as the mineral gold, known to mankind since the Neolithic times and truly valued at all times.
After all, in the whole wide world there is no such precious mountain rock in the form of native gold of a yellowish color, which would not have been mined through hard work over many centuries due to the irresistible craving for it by insane humanity.
Wherever countless hordes of labor artels of free prospectors, eager to get rich every minute, went:
- on a dedicated search for gold mines
- and the coveted prey of despicable gold,
to decorate yourself and become unspeakably rich under the bright shine of the solar metal.
Gold catalysis
It is difficult to find another substance in the world that would play such an ominous role in the history of civilization. Raised by human labor to the pedestal of a sovereign measure of value, element No. 79 became a symbol of inhumanity in class society.
Gold history of discovery properties and alloys
To please the “god of gold,” during the Middle Ages, entire nations and the great civilizations that had been created in the Western Hemisphere were completely destroyed. And today millions more people on earth live in the world of the “yellow devil,” which “surrounds a person with its web, drowns him out, sucks blood and brain, devours muscles and nerves...”
But, of course, this element No. 79 in itself is no more guilty of all these atrocities than element No. 92 is to blame for the destruction of Hiroshima.
In pursuit of the precious metal, numerous geographical and other discoveries were made, and the volume of information on the basis of which chemistry subsequently arose was significantly expanded.
For many hundreds of years, alchemists, inspired by the tempting goal of producing the “king of metals” from cheaper and more accessible materials, studied thousands of substances and reactions.

Gold history of discovery properties and alloys
In search of the “philosopher’s stone”, they carried out countless experiments, developed the technique of chemical experiments and, in particular, the separation of mixtures and solutions, and obtained many previously unknown substances - acids, alkalis, salts.
The complete collapse of centuries-old attempts to turn “imperfect” metals into gold led to the establishment of the basic concept of chemistry - the concept of a chemical element.
Financial instrument

For centuries, gold has been the most popular investment product, which is insured against any political or economic crisis. Each modern state has its own gold and foreign exchange fund with a certain reserve of this material. The total volume of reserves of all countries reaches 30,000 tons. Moreover, literally half a century ago this figure was 38 tons.
The presence of gold reserves determines economic independence, and is also an anti-crisis remedy that stabilizes the internal exchange rate of the national currency. A significant portion of gold reserves are located in the United States of America, with Germany in second place.
Currently, the use of gold in the industrial, medical and jewelry industries is very extensive. When wondering what gold is needed for, you should pay attention to the fact that the metal’s applications include the space industry, fashion trends in jewelry, and dental prosthetics.
Being an expensive material, gold continues to retain its investment, industrial and medical properties for many millennia. And it’s easy to guess that this trend will continue in the future, and the unique properties of aurum will find other niches of application.
Video: Indian legends about the Golden Cave of the Devils Tower
The first grains of gold
The first grains of gold fell into the hands of man from placers. The richest country in gold in the ancient world was Egypt. A ton of gold-bearing sand and gravel in the White and Blue Nile valleys contained up to 80 grams of gold. Precious metal was mined here five and a half thousand years ago.
Under Pharaoh Thutmes III (1501 - 1447 BC), the annual production was about 50 tons of gold! For comparison, it can be mentioned that in 1910, a record year for Tsarist Russia, 63 tons were received from all mines.
In Europe, already in ancient times, the Iberian Peninsula was famous for its gold. The content of ore gold in some quartz veins there reached 100 grams per ton of quartz, but the main source of the metal was placers in the valleys of the Tagus, Duero and other rivers, where about 1,500 tons of gold were extracted.

Gold history of discovery properties and alloys
Placer deposits were also known in the center of Europe - in the valleys of the Rhine and Rhone rivers. Ore gold was mined in Silesia, Bohemia and Hungary. In Silesia in 1211, a city arose near gold deposits, the name of which reflected its “golden” origin - Goldberg. However, in terms of the amount of gold mined, the Middle Ages cannot be compared with antiquity.
After the discovery of America, gold in Europe became more and more abundant. However, the flow of gold that poured from America to Europe was not explained by the intensified development of gold deposits in the New World, but by the robbery of gold accumulated over many centuries by the indigenous inhabitants of America. It was then, in the 16th – 17th centuries, that the Aztec and Mayan states perished at the hands of the Spanish and Portuguese conquistadors.
After robbing the Indians and exporting their gold to Europe, gold mining begins in South America. The Brazilian gold deposits of Minas Gerais and Mato Grosso, discovered in the first quarter of the century, provided 50% of the world's gold production for 50 years.
In 1848, rich placers were discovered in California, in 1851 in Australia, in 1886 in South Africa, then in Alaska.

Gold history of discovery properties and alloys
The largest foreign gold deposits being developed in our time are located in Canada, the USA, Australia, and South Africa. African deposits account for more than 50% of the world's gold production, and it is no coincidence that the most overtly repressive regime is raging in South Africa.
Over the entire period of its existence, humanity has produced, according to some estimates, approximately 50,000, according to others, about 100,000 tons of gold. Every year in the countries of the world, on average, more than a thousand tons of gold are mined. Most of it goes into the basements of banks to provide paper and metal money issued for circulation.

Gold history of discovery properties and alloys
Gold money
The first gold money was simply pieces or ingots of the precious metal. The buying and selling process was quite complicated. When going to the market, the selling party, in addition to the goods, took with them scales with weights, a hammer, a chopper and an anvil.
Having bargained with the owner of the goods, the buyer cut off a piece of a certain weight from his ingot. And since it was difficult to immediately cut off the desired piece by eye, the operation of chopping and weighing the metal had to be repeated several times.
The matter was greatly simplified when they began to put a stamp on pre-weighed pieces of gold, certifying the purity of the metal and the accuracy of the weight. Such pieces of gold were accepted without weighing - they could simply be counted.
But such money soon revealed a significant flaw: if you saw off or chop off a little gold from it, then it would be extremely difficult to detect such theft by eye - after all, money did not have any specific form.
Only after they began to give money a “standard” form and cover their entire surface with drawings and inscriptions, did the theft of gold from them lose its meaning.

Gold history of discovery properties and alloys
The first gold coins appeared 2500 - 2600 years ago in Lydia, a state that arose in the western part of Asia Minor on the ruins of the Hittite kingdom.
After the conquest of Lydia by the Persian king Cyrus, the minting of gold coins spread to other states of the Near and Middle East.
According to another version, the birthplace of the coin is considered to be the Greek island of Aegina, which was part of the possession of the Argive king Pheidon. Coins appeared here around 750 BC. e.
Pure gold is extremely soft, and coins made from it were easily worn, losing some of their value. Therefore, to increase the hardness of coins, other metals, in particular copper, began to be added to gold over time.
However, as trade relations expanded and trade turnover grew, the shortcomings of gold and metal money in general became increasingly apparent: bulkiness, heavy weight, inconvenience of storage, and many others.
Gradually, gold money gave way to paper money, which was sent as a kind of representative of gold - fused into ingots, it remained in the storerooms of banks. Thus, having left the sphere of circulation directly, gold fully retained its role as a universal equivalent of value, which even today measures everything created by human labor.
In the USSR, along with other goods, gold served as a backing medium for banknotes in circulation. One ruble is 0.987412 grams of pure gold.
Industrial significance
One of the key areas where gold is used is industrial production. The particular importance of the precious metal in this branch of human activity is due to its specific properties, namely malleability and ductility. They make it possible to create micron wire and ultra-thin foil sheets from raw materials.

Gold is also characterized by a high degree of resistance to aggressive influences, which is important for the chemical field and electronics. Moreover, even low thermal and electrical conductivity does not make gold less in demand than copper.
Among the key branches of modern industry where gold is used, the following should be highlighted :
- Transport sector.
- Chemical and petrochemical production.
- Modern nanotechnology.
- Telecommunications areas.
- Electronics and production of measuring equipment.
- Energy industry.
Aurum is an indispensable solution for welding applications when creating advanced technical samples, thermocouples or small galvanometer components.
And although gold is inferior to many platinum group metals in terms of chemical and mechanical resistance, it remains indispensable for the manufacture of electrical conductors, especially in the field of microelectronics. Currently, based on this raw material, both gold conductors and electroplating of individual boards, microcircuits and connectors are created.
Video: Mystery villa by the lake
Where is pure gold found?
What is pure gold, where is it found and obtained? The chemical element, which occupies seventy-ninth place in the periodic table, is found in nature mainly in a free state.
The largest nugget found on earth was discovered in Australia. He weighed about 112 kilograms. Natural gold usually contains impurities of other metals, most often silver (up to 20%) and copper (also up to 20%), much less often - platinum group metals.
But sometimes compounds of gold with other elements are also found, for example tellurides.
Gold is everywhere - in the earth and water, in wood, leaves, in the blood and tissues of living organisms. The total amount of gold in the seas and oceans is at least 500 million tons, in the earth's crust - more than 100 billion tons!
In bedrock deposits, gold particles ranging in size from microns to millimeters are embedded in solid rocks. When they are destroyed, gold, along with sand and clay, is carried away by water into river beds, where sometimes gold placers are formed, from which gold was mainly mined until the last century.

Gold history of discovery properties and alloys
Currently, most gold is obtained from primary deposits, extracting it from pre-ground hard rocks using cyanide compounds.
This method, which makes it possible to extract gold even from very poor ores, was proposed in 1843 by the Russian engineer and scientist Pyotr Romanovich Bagration. The crushed rock is treated with a dilute solution of sodium cyanide, in which gold dissolves well. The resulting “golden” solution is passed through finely crushed zinc, which releases gold in a free state.
To further purify gold from random impurities, it is treated with hot sulfuric acid or electrolysis is used.
In Russia, gold deposits are located in the Urals and Siberia, Transbaikalia, Yakutia, and Kolyma. Pure gold is a yellow, shiny, soft (drawn with a fingernail), very malleable metal.

Gold history of discovery properties and alloys
Gold and its properties
A piece of gold the size of a match head can be pulled into a wire 3 kilometers long or flattened into a transparent bluish-green sheet with an area of 50 square meters.
Gold is one of the heaviest metals: one cubic centimeter weighs 19.3 grams. At 1063 degrees Celsius, gold melts, and at 2713 ℃ it boils, forming yellow-green heavy steam.
Element No. 79 has exceptional chemical resistance. In air it does not change even with strong heating. Alkalies and acids do not affect it. Only a mixture of hydrochloric and nitric acids—aqua regia and, to a lesser extent, chlorine water—dissolve gold.
Gold enters into chemical reactions with difficulty; the products of these reactions easily decompose - some with slight heating, others spontaneously, and sometimes with the release of energy - an explosion.
Alchemists called gold the king of metals. But, having lost its throne, having ceased to be a treasure, gold will not lose its value - the guarantee of this is its exceptional physical and chemical properties, which make element No. 79 an important technical material.
Description of the characteristics and valuable properties of useful gold
The ore mineral natural gold itself is an inert metal, which under normal environmental conditions does not form oxides, which means it is not susceptible to oxidation and corrosion, which is why it is classified as noble, that is, precious metals.

The color of gold found in gold-bearing veins of rocks often depends on the size of the smallest particles, and a single piece of a weighty nugget is a solid solution of natural gold, consisting of such low-grade metal impurities as:
- copper and lead, iron and silver,
- bismuth and platinum, manganese and mercury,
where there is a huge variety of compressed forms of skeletal crystals, lumpy formations, in appearance somewhat reminiscent of twisted threads.
These irregularly shaped, hooked clusters in the form of native gold have a granular-crystalline particle structure, where in terms of high density this heavy metal (79 Au) ranks 7th in honorable place, after:
- rhenium (75 Re) and osmium (76 Os),
- iridium (77 Ir) and platinum (78 Pt),
- neptunium (93 Np) and plutonium (94 Pu).
In the system of chemical elements of D.I. Mendeleev, they are periodically located, sequentially following each other, according to their ordinal number in the structural configuration of the protons and electrons of the atomic nucleus. Pure gold itself is a highly plastic soft metal with remarkable thermal conductivity, capable of being noticeably deformed under the influence of external forces without much destruction, which is why, as a result of technological processes, it can easily be:
- bending and stretching,
- stamping and drawing.
And the unique ability of the thinnest sheets of gold leaf to reflect infrared light has made it an indispensable element of decorative items.
Video: The riddle of the golden stater
There is an opinion that sake gold itself is one of the least useful metals. Is it so?
An erudite engineer of the early twentieth century would answer: “Undoubtedly, so.” Engineers from the mid-sixties of the twentieth century were not so categorical. The technology of the past did without gold not only because it was too expensive. There was no particular need for properties unique to gold.
However, the statement that these properties were not used at all would be incorrect. Church domes were gilded because of the chemical resistance, high reflectivity, and ease of mechanical processing of gold. Modern technology also uses these properties.

Gold history of discovery properties and alloys
Gold and its alloys
Gold is a very soft metal, it can be easily flattened and turned into the thinnest plates and sheets. In some cases, this is very convenient. Despite this, most gold products are cast.
Melting point of gold: 1063℃. However, even the ancient masters had to make sure that it was not possible to give gold all the necessary shapes by casting. When making a regular jug, the handle had to be cast separately and then soldered.
Historians and archaeologists have found that soldering metals has been known to people for several millennia. Only the ancients soldered not with tin, but with gold, or rather, an alloy of gold and silver. Modern technology also sometimes has to use gold solder.
In terms of electrical conductivity, gold ranks third after silver and copper. When gold comes into contact with copper under pressure in a reducing environment or in a vacuum, the process of diffusion—the penetration of molecules of one metal into another—goes quite quickly.

Gold history of discovery properties and alloys
Parts made of these metals are connected to each other at temperatures significantly lower than the melting point of copper, gold or any of their alloys. Such connections are sealed with “golden seals”. They are used in the manufacture of certain types of radio components.
The strength of “golden seals” is somewhat lower than that of joints obtained by fusion, but is sufficient for radio components. Alloys of gold with silver or copper are used to make the hairs of galvanometers and other instruments, as well as electrical contacts.
These structurally simple parts take on a huge number of short circuits and open circuits. It is especially important that the contacts do not stick, so that they respond to every impulse.
In creating alloys that give the least amount of adhesion, gold plays a special role.
Alloys of gold with palladium (30%) and platinum (10%), palladium (35%) and tungsten (5%), zirconium (3%), manganese (1%) work flawlessly. Special literature describes alloys for special purposes that can compete with gold. This, for example, is an alloy of platinum with 18% iridium, but it is more expensive than any of the above.
The best contact alloys are very expensive, but modern space technology cannot do without them. They are also used in the most important non-spacecraft, which must operate especially reliably.
Application of gold in industry
Gold and its alloys have become a structural material not only for electronic parts and contacts, but also for giant particle accelerators. The accelerator, as a rule, is a huge annular chamber - a pipe rolled into a bagel.
The greater the vacuum that can be created in such a pipe, the longer the life of its elementary particles. The pipe is made of stainless steel melted in a vacuum. Its inner surface is polished to a mirror shine - the polished surface holds vacuum better.
The pressure in the particle accelerator does not exceed billionths of atmospheric pressure! It is unnecessary to explain how difficult it is to maintain a vacuum in such a giant “steering wheel.” This is all the more difficult because the “steering wheel” is only the main contour, in which there are also bends, sleeves, and joints.
O-rings and washers for accelerators are made from soft, malleable gold. The camera joints are soldered with gold. As a result, it is possible to obtain the deepest vacuum in the accelerator. In some cases, the plasticity of gold turns out to be an irreplaceable quality, in others, on the contrary, it creates difficulties.

Gold history of discovery properties and alloys
One of the oldest uses of gold is in dentures. Of course, it is easier to give a soft metal the desired shape, but a tooth made of pure gold will be at least disadvantageous - it will very quickly become wrinkled and worn out.
For the manufacture of dentures and jewelry, not pure gold is used, but its alloys with silver or copper. Depending on the silver content, such alloys differ in color: with 20 - 40% silver the metal is greenish-yellow, with 50% it is pale yellow.
These alloys are further strengthened by heat treatment. Here gold behaves very peculiarly. The process of hardening steel is well known. The metal is heated to a certain temperature and then quickly cooled. This treatment imparts hardness to the steel.
In order to remove the hardening, the metal is reheated and cooled slowly - this is annealing. Alloys of gold with copper and silver, on the contrary, acquire softness and ductility with rapid cooling, and with slow annealing - hardness and brittleness.
Alloys of gold and platinum are sometimes used to make chemical glassware and equipment.
Gilding
Gold coatings have been known since ancient times. If the pharaohs' litters were truly gold, they would be two and a half times heavier than the iron ones. Gold is one of the heaviest metals; only osmium, iridium and platinum exceed it in specific gravity.
An interesting detail: the specific gravity of tungsten is almost the same as the specific gravity of gold. In ancient times, tungsten was not known, but if we assume that the golden crown of the Syracusan king Hieron would have been counterfeited not with silver, but with tungsten, then the great Archimedes, using the law he derived, would not have been able to detect the fakes and convict the fraudulent master.
The pharaohs' stretchers were actually made of wood, covered with the finest gold foil. The thinnest sheets of gold were glued to wood, copper, and later to iron with special varnishes. On items in constant use, such gold plating lasted for about 50 years.
True, this method of gilding was not the only one. In some cases, the product was covered with a layer of special glue and sprinkled with the finest gold powder.
Since the middle of the last century, after the Russian scientist B. S. Jacobi discovered the processes of electroplating and electroplating, the old methods of gilding have almost fallen out of use. The electroplating process is not only more productive, it allows you to give the gold plating different shades.
The addition of a small amount of copper cyanide to the gold electrolyte gives the coating a red tint, and in combination with the addition of silver cyanide, it gives the coating a pink tint; By adding silver cyanide alone, you can get a greenish tint to gold coatings.
Gold coatings are highly durable and reflective. Nowadays, various parts of conductors in high-voltage radio equipment and parts of X-ray machines are gilded. Reflectors are made with gold coating for drying by infrared rays.
The surfaces of several artificial Earth satellites were gold-plated. The goal here was to protect them from the effects of corrosion and excess heat.
The newest method of applying gold coatings is cathode sputtering. An electric discharge in a discharged gas is accompanied by destruction of the cathode. Its particles fly at enormous speed and can be deposited not only on metal, but also on other materials: paper, wood, ceramics, plastic.
This method makes it possible to obtain the finest gold coatings. It is used in the manufacture of photocells, special mirrors and in some other cases.

Gold history of discovery properties and alloys
Paints of gold
The “nobility” of gold extends only to certain limits. In other words, it is possible to obtain its connections with other elements. The industrial process of extracting gold from ores - cyanidation - is based on the interaction of gold with alkali metal cyanides:
4Au+8KCN+2H2O = 4K (Au (CN)2)+4KON
The basis of another important process - chlorination (it is now used not so much for extraction as for refining - gold purification) is the interaction of gold with chlorine.
Sometimes there are ores in which gold is not in a free state, but in combination with tellurium or selenium. Some gold compounds have industrial applications. First of all, it is gold chloride AuCl3, formed by dissolving gold in aqua regia.
Using this compound, high-quality red glass is obtained - golden ruby. It was first made at the end of the 17th century by Johann Kunkel, but a description of the method for its production appeared only in 1836.
A solution of gold chloride is added to the mixture during the cooking process, and by changing the dosage, different shades of glass are obtained from soft pink to dark purple. The best colors for glass are those that contain lead oxide. True, in this case, another component has to be introduced into the charge - a clarifier: 0.3 - 1.0% “white arsenic” As2O3.
Coloring glass with gold compounds is not very expensive - for uniform, intense coloring of the entire mass, only 0.001 - 0.003% AuCl3 is needed. You can also give glass a red color by introducing copper or selenium compounds into the charge. They are certainly cheaper than gold compounds, but working with them and obtaining high-quality products is much more difficult.
The production of “copper ruby” is complicated by the variability of color; the shade greatly depends on the cooking conditions.
In the production of “selenium ruby,” difficulties arise from the burnout of selenium and sulfur from cadmium sulfide, which is also introduced into the charge.
“Golden Ruby” does not lose its bloom during high-temperature treatment. Its undeniable advantage is that unsuccessful cooking in this case can be corrected by subsequent remelting.
As a coloring agent, gold chloride is also used when painting on glass and porcelain. In addition, it has long been used as a toning agent in photography. “Gold Fixer” gives photo prints black-violet, brown or purple-violet shades.
For the same purposes, another gold compound is sometimes used - sodium chloraurate NaAuCl4.

Gold history of discovery properties and alloys
Gold in medicine
The first attempts to use gold for medicinal purposes date back to the times of alchemy. They were little more successful than the search for the philosopher's stone. In the 16th century, Paracelsus tried to use gold preparations to treat certain diseases, in particular syphilis.
“The transformation of metals into gold should not be the goal of chemistry, but the preparation of medicines,” he wrote. Much later, compounds containing gold were proposed as a medicine against tuberculosis.
It would be wrong to assume that this proposal was without foundation: in vitro, that is, outside the body, “in a test tube,” these salts had a detrimental effect on the tuberculosis bacillus, but to effectively combat the disease, fairly high concentrations of these salts are needed.
Nowadays, gold salts are important for the fight against tuberculosis only insofar as they increase resistance to the disease.
It was also found that gold chloride in concentrations of 1:30,000 begins to inhibit alcoholic fermentation, when the concentration increases to 1:3000 it significantly inhibits it, and in a concentration of 1:200 it completely stops.
A more effective medical remedy turned out to be gold and sodium thiosulfate AuNaS2O3, which is successfully used to treat a difficult-to-treat skin disease - erythematous lupus.
Organic gold compounds, primarily crizolgan and triphal, were not used in medicine.
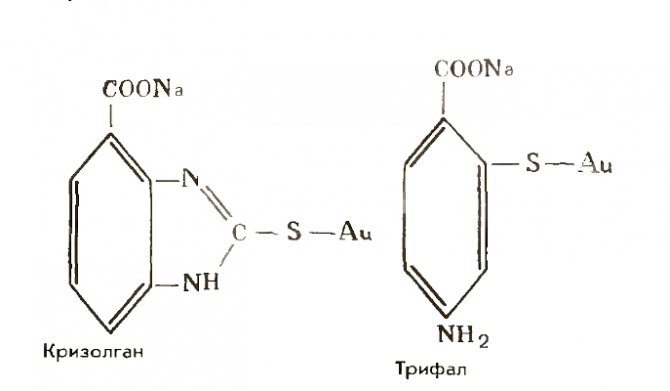
Gold history of discovery properties and alloys
At one time in the middle of the 20th century, Krizolgan was widely used in Europe to combat tuberculosis, triphal - as a less toxic and more effective medicine for erythematous lupus than gold and sodium thiosulfate.
The highly active drug krizanol was synthesized in the Soviet Union:
(Au - S - CH2 - CHOH - CH2SO3 )2Ca - for the treatment of lupus and bone tuberculosis. After the discovery of radioactive isotopes of gold, its role in medicine increased significantly.
Colloidal particles of these isotopes are used to treat malignant tumors. These particles are physiologically inert, and therefore it is not necessary to remove them from the body. Injected into specific areas of the tumor, they irradiate only the affected areas.
Radioactive gold can cure some forms of cancer. A special “radioactive pistol” has been created, the clip of which contains 15 rods of radioactive gold with a half-life of 2.7 days. Practice has shown that treatment with “radioactive needles” makes it possible to eliminate a superficial breast tumor already on the 25th day.

Gold history of discovery properties and alloys
Gold catalysis
Radioactive gold has found application not only in medicine. Opportunities have arisen to use it as a catalyst in several important petrochemical and chemical processes instead of platinum.
Particularly interesting are the prospects for using the catalytic properties of gold in the field of high-speed flights. It is known that at an altitude of 80 kilometers and above there is quite a lot of atomic oxygen in the atmosphere.
The combination of individual oxygen atoms into an O2 molecule is accompanied by the release of a large amount of heat. Gold catalytically accelerates this process.
It is very difficult to imagine an ultra-high-speed aircraft that operates practically without fuel, but theoretically such a design is possible. The engine will work thanks to the energy released during the dimerization reaction of atomic oxygen.
Having risen to an altitude of 80 kilometers (this is significantly higher than the ceiling of modern aircraft), the pilot turns on the oxygen-catalytic engine, in which atmospheric oxygen comes into contact with the catalyst.

satellite with a gold plate. Message to Civilizations on a Gold Plate
Of course, it is still difficult to judge the characteristics of such an engine, but the idea is very interesting and apparently not fruitless. On the pages of scientific journals, foreign experts discussed possible designs of the catalytic chamber and proved the inappropriateness of using a finely dispersed catalyst.
All this indicates the seriousness of intentions. In the meantime, such engines will be used not on airplanes, but on rockets, and perhaps further research will bury this idea as impracticable.
But this fact, like everything discussed above, shows that the time has come to abandon the established view of gold as a metal useless for technology.
Artificial acquisition
It is obtained in many ways, for example: 1) by precipitation from gold solutions with iron sulfate, hydrogen peroxide, sulfurous acid, amyl alcohol, etc.; 2) electrolysis. The precipitation of gold from solutions with natural sulfides (pyrite, galena, sphalerite, chalcopyrite), calcite, siderite, etc. at different temperatures was artificially reproduced. Co-precipitation of gold and sulfides from solutions has been studied.
Diagnostic signs
Similar minerals. Can be confused with chalcopyrite, pyrite, millerite.
The main diagnostic features of gold: golden yellow color, low hardness (easily cut with a knife), high malleability and density, inability to oxidize in air. In small segregations, gold can be mixed with pyrite, chalcopyrite, and millerite. It differs from them in less hardness, lack of tarnish, and malleability. In polished sections under a microscope, it is easily distinguished from chalcopyrite in reflectivity and in its reaction with AgNO3 (does not turn black); differs from native silver in its greater reflectivity in blue light by blackening from KCN; from tellurides - isotropy.
Associated minerals. Quartz, sulfides, chalcedony, calcite, ankerite, siderite, rhodochrosite, adularia, barite, fluorite, zeolites, magnetite, zircon, cassiterite, ilmenite, platinum and osmic iridium.

In quartz











