Iridium, properties of the atom, chemical and physical properties.
Ir 77 Iridium
192.217(3) 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d7 6s2
Iridium is an element of the periodic system of chemical elements of D.I. Mendeleev with atomic number 77. It is located in the 9th group (according to the old classification - a secondary subgroup of the eighth group), the sixth period of the periodic system.
Iridium atom and molecule. Iridium formula. Structure of the iridium atom
Isotopes and modifications of iridium
Properties of iridium (table): temperature, density, pressure, etc.
Physical properties of iridium
Chemical properties of iridium. Iridium interaction. Chemical reactions with iridium
Obtaining iridium
Applications of iridium
Table of chemical elements D.I. Mendeleev
Silver market
Global silver demand is forecast to reach 1 billion ounces in 2021, up 11% year-on-year and at an eight-year high, driven primarily by industrial end users. Industrial demand is estimated to grow 9% year-on-year to 510 million ounces this year (Source: The Silver Institute), and the introduction of 5G technology in consumer electronics is expected to boost silver sales.
However, the surplus in the silver market is expected to remain, which will limit the price upside to some extent. Silver traded sideways before falling, but it outperformed gold last week.
Iridium atom and molecule. Iridium formula. Structure of the iridium atom:
Iridium (Latin Iridium, from ancient Greek ἶρις - “rainbow”) is a chemical element of D. I. Mendeleev’s periodic system of chemical elements with the designation Ir and atomic number 77. Located in the 9th group (according to the old classification - secondary subgroup of the eighth group), the sixth period of the periodic table.
Iridium is a metal. Belongs to the group of transition metals, as well as precious metals and platinum group metals.
As a simple substance, iridium under normal conditions is a very hard, refractory, silvery-white metal.
The iridium molecule is monatomic.
Chemical formula of iridium Ir.
The electronic configuration of the iridium atom is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d7 6s2. The ionization potential (first electron) of the iridium atom is 865.19 kJ/mol (8.96702(22) eV).
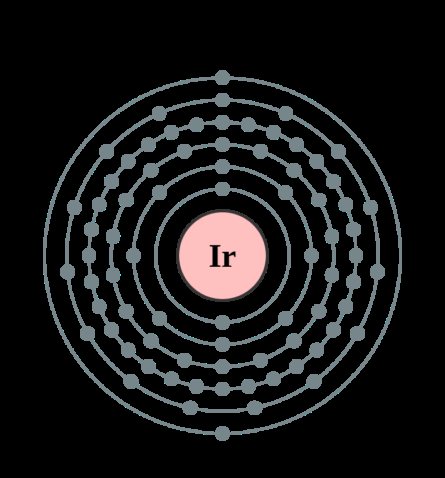
Structure of the iridium atom. The iridium atom consists of a positively charged nucleus (+77), around which 77 electrons move through six shells. In this case, 75 electrons are in the internal level, and 2 electrons are in the external level. Since iridium is located in the sixth period, there are only six shells. First, the inner shell is represented by the s-orbital. The second - the inner shell is represented by s- and p-orbitals. The third and fifth – inner shells are represented by s-, p- and d-orbitals. The fourth - the inner shell is represented by s-, p-, d- and f-orbitals. The sixth - outer shell is represented by the s-orbital. At the internal energy level of the iridium atom in the 5d orbital there are four paired electrons and three unpaired electrons. At the outer energy level of the iridium atom, there are two paired electrons in the 6s orbital. In turn, the nucleus of an iridium atom consists of 77 protons and 115 neutrons. Iridium belongs to the elements of the d-family.
The radius of the iridium atom (calculated) is 180 pm.
The atomic mass iridium atom is 192.217(3) a. eat.
Iridium, along with osmium, has the highest density among all simple substances.
Iridium is the rarest metal on Earth. Its content in the earth's crust is 4.0×10-8%.
Iridium, properties of the atom, chemical and physical properties
Use, application of metal
The production of crystals is not complete without crucibles made of precious metal.
It is used in precision instrument parts and as a coating for electrical contacts.
In long-life spark plugs they are used as electrodes.
The advantage of such spark plugs is the lightning-fast acceleration of the engine and its stable operation. There is no risk of loss of spark; such spark plugs have excellent anti-corrosion properties. There is only one drawback - the price...
Metal is used for the “eternal” nibs of fountain pens.
Economical: in some countries, instead of expensive iridium, they use an alloy of niobium with rhenium - it is almost as wear-resistant, but in appearance it differs little from iridium.
Iridium is an excellent catalyst, like all its platinum metal relatives. However, the high price limits its use.
Organic synthesis is now impossible without iridium catalysts. Work on organic synthesis received the Nobel Prize in Chemistry. To make it clear, organic synthesis will make it possible to switch to “green chemistry”, abandon the use of fossil resources, and switch to renewable resources.
The kilogram standard is created from a platinum-iridium alloy and is stored in France.
Informative: don’t argue if you see in Russia, at the All-Russian Research Institute of Metrology, the international standard of the kilogram. 6 such copies of that French standard were made.
An alloy of iridium and titanium is used in deep-sea pipelines.
The Ir-192 isotope is used in flaw detectors and thickness gauges (portable).
Fabulous prospects for iridium in medicine
Pacemakers use an iridium-platinum alloy.
Oncologists use the iridium isotope Ir-192 as a source of gamma radiation. It is used to treat breast and prostate cancer (in the early stages of the disease).
Methods have been developed for the treatment of epilepsy, Parkinson's disease, and schizophrenia by introducing iridium electrodes into the brain. The method of implanting microelectrodes opens up bright prospects for creating prosthetic eyes and hearing aids.
Properties of iridium (table): temperature, density, pressure, etc.:
Detailed information on the website ChemicalStudy.ru
| 100 | General information | |
| 101 | Name | Iridium |
| 102 | Former name | |
| 103 | Latin name | Iridium |
| 104 | English name | Iridium |
| 105 | Symbol | Ir |
| 106 | Atomic number (number in table) | 77 |
| 107 | Type | Metal |
| 108 | Group | Precious, transition metal, platinum group metal |
| 109 | Open | Smithson Tennant, Great Britain, 1803 |
| 110 | Opening year | 1803 |
| 111 | Appearance, etc. | Very hard, refractory, silvery-white metal |
| 112 | Origin | Natural material |
| 113 | Modifications | |
| 114 | Allotropic modifications | |
| 115 | Temperature and other conditions for the transition of allotropic modifications into each other | |
| 116 | Bose-Einstein condensate | |
| 117 | 2D materials | |
| 118 | Content in the atmosphere and air (by mass) | 0 % |
| 119 | Content in the earth's crust (by mass) | 4,0·10-8 % |
| 120 | Content in seas and oceans (by mass) | |
| 121 | Content in the Universe and space (by mass) | 2,0·10-7 % |
| 122 | Abundance in the Sun (by mass) | 2,0·10-7 % |
| 123 | Content in meteorites (by mass) | 0,000054 % |
| 124 | Content in the human body (by weight) | |
| 200 | Properties of the atom | |
| 201 | Atomic mass (molar mass) | 192.217(3) a. e.m. (g/mol) |
| 202 | Electronic configuration | 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d7 6s2 |
| 203 | Electronic shell | K2 L8 M18 N32 O15 P2 Q0 R0
|
| 204 | Atomic radius (calculated) | 180 pm |
| 205 | Empirical atomic radius* | 135 pm |
| 206 | Covalent radius* | 141 pm |
| 207 | Ion radius (crystalline) | Ir 3+ 82 (6) pm, Ir 4+ 76.5 (6) pm, Ir 5+ 71 (6) pm (in parentheses the coordination number is indicated - a characteristic that determines the number of nearest particles (ions or atoms) in a molecule or crystal) |
| 208 | Van der Waals radius | |
| 209 | Electrons, Protons, Neutrons | 77 electrons, 77 protons, 115 neutrons |
| 210 | Family (block) | d-family element |
| 211 | Period in the periodic table | 6 |
| 212 | Group on the periodic table | 9th group (according to the old classification - a secondary subgroup of the 8th group) |
| 213 | Emission spectrum | |
| 300 | Chemical properties | |
| 301 | Oxidation states | -3, -1, 0, +1, +2, +3, +4, +5, +6, +7, +8, +9 |
| 302 | Valence | I, II, III, IV, V, VI |
| 303 | Electronegativity | 2.2 (Pauling scale) |
| 304 | Ionization energy (first electron) | 865.19 kJ/mol (8.96702(22) eV) |
| 305 | Electrode potential | Ir3+ + 3e– → Ir, Eo = +1.0 V |
| 306 | Electron affinity energy of an atom | 151 kJ/mol |
| 400 | Physical properties | |
| 401 | Density* | 22.56 g/cm3 (at 20 °C and other standard conditions , state of matter – solid), 19 g/cm3 (at melting point 2446 °C and other standard conditions , state of matter – liquid) |
| 402 | Melting temperature* | 2446 °C (2719 K, 4435 °F) |
| 403 | Boiling temperature* | 4130 °C (4403 K, 7466 °F) |
| 404 | Sublimation temperature | |
| 405 | Decomposition temperature | |
| 406 | Self-ignition temperature of a gas-air mixture | |
| 407 | Specific heat of fusion (enthalpy of fusion ΔHpl)* | 41.12 kJ/mol |
| 408 | Specific heat of evaporation (enthalpy of boiling ΔHboiling)* | 564 kJ/mol |
| 409 | Specific heat capacity at constant pressure | 0.131 J/g K (at 0-100 °C) |
| 410 | Molar heat capacity | 25.1 J/(K mol) |
| 411 | Molar volume | 8.54 cm³/mol |
| 412 | Thermal conductivity | 147 W/(mK) (at standard conditions ), 147 W/(mK) (at 300 K) |
| 500 | Crystal cell | |
| 511 | Crystal grid #1 | |
| 512 | Lattice structure | Cubic face centered
|
| 513 | Lattice parameters | 3.840 Å |
| 514 | c/a ratio | |
| 515 | Debye temperature | 430 K |
| 516 | Name of space symmetry group | Fm_3m |
| 517 | Symmetry space group number | 225 |
| 900 | additional information | |
| 901 | CAS number | 7439-88-5 |
Note:
205* The empirical radius of iridium according to [1] and [3] is 136 pm.
206* The covalent radius of iridium according to [1] and [3] is 141±6 pm and 127 pm, respectively.
401* The density of iridium according to [3] and [4] is 22.65 / 22.56±0.01 g/cm3 (at 0 °C and other standard conditions , the state of matter is a solid) and 22.562 g/cm3 (at 20 °C and other standard conditions , the state of matter is a solid) respectively.
402* The melting point of iridium according to [3] and [4] is 2466 °C (2739 K, 4471 °F) and 2450 °C (2723.15 K, 4442 °F), respectively.
403* The boiling point of iridium according to [3] and [4] is 4428 °C (4701 K, 8002 °F) and 4380 °C (4653.15 K, 7916 °F), respectively.
407* The specific heat of fusion (melting enthalpy ΔHmelt) of iridium according to [3] and [4] is 26.0 kJ/mol and 26.4 kJ/mol, respectively.
410* The specific heat of evaporation (boiling enthalpy ΔHboil) of iridium according to [3] and [4] is 610 kJ/mol and 612.5 kJ/mol, respectively.
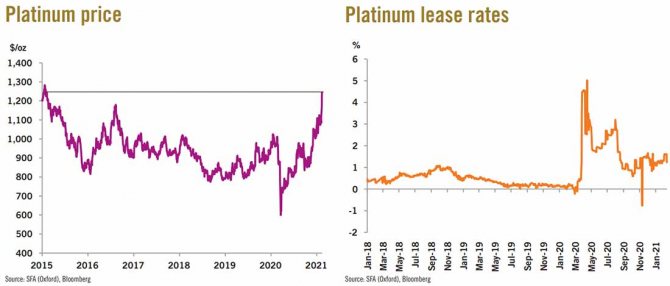
Platinum market
Last week, the price of platinum rose to a six-year high. From a technical perspective, the price has broken through the 2016 high and is now heading toward potential resistance at $1,290/oz. A price rally may be the result of a temporary reduction in liquidity in the market. The closure of the Anglo American Platinum plant last year resulted in reduced production of refined platinum, and additional market purchases by Anglo have further reduced the availability of the metal.
However, rental rates have fallen since a sharp jump in April last year, confirming that long-term supply should not be a concern. Despite supply disruptions in South Africa, weak industrial demand has kept the market surplus in 2021 and is expected to remain so for the foreseeable future.
Investment in platinum helped absorb much of the surplus in 2020, so if this level of demand continues into 2021, the price could gain support.