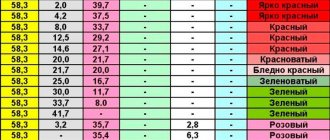
Almost every consumer has a Soviet TV or radio at home. Throwing away this equipment, no one even imagines what value it may contain. After all, in old electronics there are parts that contain noble substances. For example, gold, silver, palladium in radio components.
Knowledgeable people do not throw such items into a landfill, but use them as a real “gold mine”. But the number of expensive metals in parts depends on the purpose of the device and the type of capacitor. Everywhere in the old devices of the former USSR, silver, gold, and palladium were used.
This was done to increase their service life. What was it like for the craftsmen not to know about this! Many “bitten their elbows” because they did not have such information.
Modern household devices do not have this “joy”. There is nothing valuable in them. Therefore they are short-lived. Only some industries continue to add Pd and platinum to equipment. Gold and silver are no longer used.
History of discovery
The history of palladium begins in 1803. Then mineral and precious metals dealer Forstner received a letter offering to sell a small amount of a new chemical element. A palladium bar was included with the letter.
The merchant put the metal up for sale. It quickly attracted the attention of buyers. The first to become interested in the new product were English chemists. They began to argue about whether the metal was a new chemical element or whether the seller was passing it off as a new product, but in fact it was an alloy known to science.
One of the scientists, chemist Richard Chenevix, bought the bar to try to expose the seller. After several experiments, he wrote a report which he presented to the Royal Society of London. In it, he wrote that this ingot was not a new chemical element, but a common compound of mercury and platinum. The secretary of this community did not believe Chenivix’s words and expressed the opinion that none of the scientists of the Royal Society could isolate a similar type of metal from platinum or mercury. The controversy intensified again. Offers of rewards have appeared for the person who can obtain this type of metal.
The scientist William Wollaston made his own report at a meeting of the Royal Society of London in 1804. He claimed that he was able to discover two new elements in platinum ore that was mined in South America. These were rhodium and palladium. After some time, the scientist admitted that it was he who created the hype around the new chemical element in order to attract attention to it and make the discovery significant.
Palladium in vitro (Photo: Instagram / chemical_elements)
Palladium
Palladium is a chemical element, metal. Atomic number – 46. Atomic mass – 106.42(1) amu. Denoted by the symbol Pd (from the Latin Palladium).
The element belongs to the transition metals and noble metals of the platinum group (light platinoids). Palladium is the lightest element of the platinum group, one of the four precious metals that can be hallmarked.
The simple substance palladium under normal conditions is a ductile metal of silvery-white color.
The metal is named after the asteroid Pallas, discovered by the German astronomer Olbers in 1802, shortly before the discovery of palladium. In turn, the asteroid is named after Pallas Athena from ancient Greek mythology. Palladium or Palladium is a legendary wooden image of Pallas Athena that fell from the sky. According to the prophecy of Helen (son of Priam), Troy will remain indestructible as long as this talisman is kept within its walls. According to legend, Troy fell only after Odysseus and Diomedes stole Palladium during a night raid.
Physical properties Palladium is a transition metal. Under normal conditions, it forms crystals of silvery-white color of cubic system, space group Fm3m, cell parameters a = 0.38902 nm, Z = 4, structural type of copper.
Palladium is plastic; microadditives of nickel, cobalt, rhodium or ruthenium improve the mechanical properties of Pd and increase hardness.
Insoluble in water. Density - 12.02 (20°C, g/cm³); under special conditions it forms colloidal palladium and palladium black. Melting point is 1554°C, boiling point is about 2940°C. The heat of fusion is 16.7 kJ/mol, the heat of evaporation is 353 kJ/mol. Specific heat capacity at 20°C - 25.8 J/(mol K), electrical resistivity at 25°C - 9.96 μOhm/cm; thermal conductivity - 75.3 W/(m K). Vickers hardness 37...39. Brinell hardness 52 kgf/mm².
Temperature coefficient of linear expansion 1.17·10−5 K−1 (in the range of 0...100°C).
The surface tension coefficient of liquid palladium at the melting point is 0.015 N/cm.
Palladium is paramagnetic; its magnetic susceptibility is +5.231·10−6 (at +20°C).
Actively absorbs hydrogen, forming solid solutions (up to 900 volumes of hydrogen per volume of Pd), while the lattice constant increases. Hydrogen is removed from palladium by heating to 100°C in a vacuum.
Chemical properties Palladium is the most chemically active of the platinum metals. At room temperature, palladium reacts with aqua regia and wet chlorine and bromine. Reacts with hot concentrated sulfuric and nitric acids, unlike other platinum metals. It can be brought into solution by anodic dissolution in hydrochloric acid. When heated, it reacts with fluorine, sulfur, selenium, tellurium, arsenic and silicon. It oxidizes when fused with potassium hydrogen sulfate, and also interacts with molten sodium peroxide. When heated in air, it is stable up to ~300°C and above 850°C. In the range of 300-850°C it dims due to the formation of a film of palladium oxide PdO, which decomposes at higher temperatures. The metal does not react with water, dilute acids, alkalis, or ammonia solution.
Isotopes Natural palladium consists of six stable isotopes: 102Pd (1.00%), 104Pd (11.14%), 105Pd (22.33%), 106Pd (27.33%), 108Pd (26.46%) and 110Pd (11.72%). The longest-lived artificial radioactive isotope is 107Pd (T1/2 7·106 years).
History of the discovery In 1803, the famous London mineral merchant Forster received an anonymous letter asking him to try to sell a small amount of a new chemical element, “palladium,” an ingot of which was enclosed with the letter. The unknown metal was put up for sale and attracted everyone's attention, and a debate broke out among English chemists: whether the metal was truly a new chemical element or an alloy of previously known metals. In order to expose the “fraud,” chemist Richard Chenevix bought an ingot of palladium and soon made a presentation to members of the Royal Society of London, where he announced that the substance was just an alloy of platinum and mercury. The Secretary of the Royal Society, chemist William Hyde Wollaston, publicly doubted Chenevix's conclusions, since other chemists had failed to isolate either platinum or mercury in this “alloy.” The controversy flared up again, and as it began to subside, an anonymous advertisement appeared in the scientific journal Nicholson's Journal that a reward of £20 would be paid to anyone who could produce artificial palladium within a year. Interest in the metal surged again, but no one managed to “make” it.
In 1804, William Wollaston reported to the Royal Society that he had discovered new previously unknown metals - palladium and rhodium - in platinum ore from South America. In an effort to purify the “raw” platinum isolated from ore from impurities of gold and mercury, he dissolved it in aqua regia, and then precipitated it from the solution with ammonia. The remaining solution had a pink tint, which could not be explained by the presence of gold and mercury. Adding zinc to the solution led to the formation of a black precipitate. Wollaston discovered that if you try to dissolve this dried sediment with aqua regia, some of it will dissolve and some will not. After diluting the solution with water, Wollaston added potassium cyanide to it, which led to a heavy precipitation of an orange-colored precipitate, which, when heated, first acquired a gray color and then fused into a droplet of metal - palladium, which in specific gravity was less than mercury. From the remaining undissolved part of the sediment, he isolated another metal - rhodium.
In February 1805, Nicholson's Journal published an open letter from Wollaston in which he admitted that the palladium scandal was his doing. It was he who put the new metal on sale, and he also gave an anonymous advertisement promising a bonus for its artificial production, already having proof that palladium was a new metal.
Occurrence and mining Palladium is one of the rarest elements in the earth's crust. Its clarke number is 1·10−6%. The metal is found in native form (allopalladium), in the form of intermetallic minerals (palladium platinum, stannopalladinite, etc.) and other compounds (palladite, braggite, etc.). In total, about 30 palladium minerals are known. Palladium accompanies other platinum metals; its content in the mixture of platinum group metals in various deposits ranges from 25 to 60%. According to the Holschmidt geochemical classification of elements, like all platinoids, it belongs to the siderophiles, that is, it has an affinity for iron and is concentrated in the Earth’s core.
Palladium is mined from primary and placer deposits. In primary deposits, the metal is part of minerals and is extracted as a by-product during the processing of nickel or copper ores. Placer deposits are permitted bedrock ore deposits where Pd has been released and accumulated as nuggets.
Palladium mining from alluvial deposits accounts for about 2% of the global production of the element. The largest of them are located in the Ural and Far Eastern regions of Russia, Canada, the USA, Australia and Colombia. The remaining 98% of Pd is extracted from the bowels of the earth at primary deposits of copper-nickel, platinum and chromium ores.
The world leaders in palladium mining from such deposits are Russia and South Africa. The undisputed first place among the mining enterprises of the industry is occupied by MMC Norilsk Nickel, producing more than 40% of the world's Pd volumes. The metallurgical plant extracts metal as a by-product during the extraction of its main products - copper and nickel. Among the mining assets of Norilsk Nickel, which have the potential for palladium reserves, the deposits on the Taimyr Peninsula - Talnakhskoye, Oktyabrskoye and Norilsk-1 - stand out. The largest known palladium deposit (not developed) is located on the territory of the Kola Peninsula in the Fedorovo-Pansky intrusive massif (Murmansk region).
Preparation Palladium is obtained mainly from the processing of sulfide ores of nickel, silver and copper. About 10% of global production is metal recovery from recycled materials.
From a solution of a mixture of noble metals in aqua regia after the precipitation of gold and platinum, dichlorodiammine palladium Pd(NH3)2Cl2 is precipitated, purified by recrystallization from an ammonia solution of HCl, decomposed to powdered palladium by calcination in a reducing atmosphere, and then melted.
By reducing solutions of palladium salts, palladium black is obtained - finely crystalline palladium powder.
Compact metal palladium is also obtained by electrodeposition from nitrite and phosphate acid electrolytes.
Application Palladium is the most affordable precious metal of the platinum group. This quality is the ticket for its use in all industries. The cost of the metal in comparison with some other precious metals is lower, with the same set of useful properties that are in demand in many areas of industry.
The physical and chemical properties of palladium allow it to be widely used in the chemical industry and electronics, and its status as a precious material allows it to be used as a raw material for jewelry.
In terms of volume of use, palladium is ahead of all other platinum group metals. The main consumer is the automotive industry. Concerns and companies producing motor vehicles use 65% of the mined metal. In this industry, almost all palladium is spent on the production of catalysts. It forms a thin coating on their inner surface that can clean car exhaust.
The catalytic properties of palladium are also valued in the chemical industry. This metal is an accelerator of various reactions and processes. With its help, the synthesis of many organic compounds occurs. Palladium catalysts are excellent purifiers of hydrogen and oxygen from excess components. Precious metal is the starting material for the manufacture of chemical glassware and equipment. The chemical industry consumes 5% of global palladium production.
15% of palladium goes to the electronics industry. Here, metal is applied to electrical contacts to improve their corrosion resistance. Alloys of palladium with rhodium, gold and platinum are used to make thermocouples and temperature regulators. When combined with silver, the metal is used to make contacts.
Palladium alloys are also used in traditional medicine. They are indispensable in dental practice, in maxillofacial surgery, in the manufacture of pacemakers and surgical instruments. Palladium is also a metal that can restore health. Its dosage forms, along with platinum, kill cancer cells and get rid of cancer. At the same time, palladium has lower toxicity than platinum. The share of palladium used in the medical industry is 5%.
In jewelry production, metal is mostly used in the form of additives to other precious metals. In the alloy, gold receives a white color thanks to palladium. Precious palladium can impart white color to six times the amount of gold. Palladium jewelry is also made. The 950 alloy contains 95% palladium and 5% ruthenium. Palladium products have a sophisticated look and are lower in cost than traditional white gold. In addition, the jewelry industry uses alloys of palladium and indium, which take on different colors from golden to lilac.

About 5% of the precious metal is used as an investment product. Palladium can be bought and sold on exchanges, you can open a palladium metal account in a bank, etc.
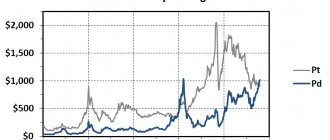
Palladium consumption in 2007 was 107 tons in the automotive industry, 40 tons in the electronics industry, and 12 tons in the chemical industry.
Supplies of palladium in the world in 2007 amounted to 267 tons (including Russia - 141 tons, South Africa - 86 tons, USA and Canada - 31 tons, other countries - 9 tons).
Prepared by Evgeniy Lavrinenko (SM)
Reserves and deposits
In its pure form, palladium nuggets cannot be found in nature. Particles of this metal are extracted along with other minerals. There can be more than 30 such compounds in total. By their appearance, grains of palladium can easily be confused with platinum. Some deposits contain both of these metals at the same time, which are mined together. Occasionally veins of palladium intersect with gold.
The main source of the appearance of this metal on planet Earth is cosmic fragments of meteorites. It is in them that a large number of crystals of this precious metal are found.
Nugget of gold (Photo: Instagram / in_sochi)
Production
Precious metal is one of the rarest elements found in the earth's crust. It is found both in the form of nuggets and mixed with other metals, often platinum, copper, gold or silver. Palladium is mined from primary and placer deposits. More often on indigenous ones.

Most of the metal is concentrated in Russia (approx. 43%), the rest in South Africa, Canada, Zimbabwe, Australia and America. The largest Russian deposit is located in Norilsk. It also provides almost half of the world's metal production.
The issue of palladium reserves is considered a state secret. By presidential decree of November 30. 1995 Information on state metal reserves is considered classified.
Mining and industrial production
The production of pure metal is carried out in several stages:
- Preparation of consumables. It is heated in special porcelain cauldrons. During the heating process, the boilers are filled with aqua regia.
- Transferring platinum into sediment using special filters. Additives and third-party components that are interesting for the production of palladium are separated from the base.
- Filtrate treatment. A refining procedure is carried out, during which a sparingly soluble compound is obtained, which is called palladium dichlorodiammine.
- Purification of the desired metal from impurities.
- Calcination. It is carried out in sealed chambers filled with hydrogen.
The resulting sponge palladium is melted into blanks of the required size and shape.
Ore mining (Photo: Instagram / khabkrai)
Ways to isolate metal
There are several ways to isolate Pd from microcircuits and capacitors.
The most popular method remains using the electrolysis process, or as a result of creating a whole chain of chemical reactions.
So, these are the methods:
- The metal is removed using an electrolytic reaction. It is created in saturated sulfuric acid. The very base of the copper part remains intact. As a result of the reaction, it is not Pd that is created, but only its compound, which is then dissolved in aqua regia. Sulfuric acid acts as an electrolyte, the part as an anode, and lead as a cathode. The voltage is left at 12 V. It is started until the part is immersed in the prepared mixture. Palladium forms as black powder or flakes. Do not allow the electrolyte to become too hot. If this happens, it must be cooled. When all the palladium has been removed, the solution should be replaced with a new one. The sediment is treated with aqua regia.
- If there is an alloy of palladium with another element, hydrochloric acid and ammonia solution are required to remove it. These substances separate Pd from other elements. The element is perfectly soluble in so-called aqua regia and nitric acid. To determine for sure the palladium content of a substance, it is necessary to monitor its color during the reaction. If the color changes to brown, then Pd is there and you can continue. Pd with gold is dissolved using nitric acid; with silver - using aqua regia. Then water is poured on top and the mixture is left for 24 hours. Then the silver chloride is filtered. This is done to ensure that only palladium and gold remain in the solution.

- Then ammonia is added to the solution in such an amount that there is an excess of it, and everything is left as is for 48 hours. Then the next stage of filtration begins. This time the gold is filtered out so that only palladium remains. The Ag precipitate is placed in hydrochloric acid, where it is reduced with zinc. Palladium is processed differently: hydrochloric acid is added to the filtrate. The result is a reaction that creates ammonium tetrachloropalladate. The precipitate is filtered for several hours, dried and calcined at high temperature (500 degrees Celsius). The result is a palladium refining product in powder form. It happens that palladium sulfide is obtained. In this case, it needs to be fused. At high temperatures, the substance will again be restored to its metallic state.
Properties and characteristics
Palladium is used in the manufacture of jewelry and parts for radio electronics. It is used in various fields of activity.
Physical
Properties:
- The atomic number in the periodic table is 46.
- Density - 12.6 g/cm3.
- Specific heat capacity - 20 °C 0.0586 cal/ (g.deg).
- Melting point - 1554°C.
- Electrical resistivity indicator - 25 °C 9.96 μOhm cm.
- Boiling point - 2940°C.
- Hardness on the Brinell scale - 49 kgf/mm2.
- Thermal conductivity index is 0.161 cal/(cm.sec.deg).
- Maximum elongation at break is up to 30%.
- The thermal expansion coefficient is 11.67•10-6.
- Maximum tensile strength is 18.5 kgf/mm2.
To increase the hardness of palladium, cold working must be performed. The indicator will increase by 2.5 times. If annealing is carried out, the hardness will be reduced.
Palladium bars (Photo: Instagram / den.electro)
Chemical
Palladium:
- Does not react with water, alkalis, diluted acids, ammonia hydrate.
- Begins to oxidize after heating to 350°C. The surfaces are covered with a dense oxide film. When heated to 850°C, it decomposes into oxygen and metal.
- When heated above 500 degrees, it reacts with strong oxidizing agents.
Palladium increases the resistance of various alloys to rust. After adding 1% palladium, resistance to the destructive effects of hydrochloric and sulfuric acid increases.
Sulfuric acid (Photo: Instagram / lina_malina_artist)
Where is palladium used?
Areas of application:
- Hydrogen technologies. Membranes that are needed to produce ultrapure hydrogen are made from this metal.
- Medicine. Medical instruments, dentures, pacemakers, and cytostatic drugs are made from palladium.
- Production of chemical glassware, parts for the assembly of high-precision measuring instruments.
- Manufacturing of precision mechanical tools.
- Production of chemical equipment used to produce hydrofluoric acid.
- Manufacturing of electrical contacts, ceramic capacitors.
- Palladium chloride is used in electroplating.
- The use of metal as a catalyst in scientific experiments. Its chloride is needed to detect the presence of small amounts of carbon monoxide in the air.
- In jewelry, palladium is an alloying component for the production of white gold. You can also find jewelry on sale in which this precious metal is the main component. It is often combined with silver in a ratio of 50x50 and with platinum in a ratio of 85 to 15.
Since palladium is a precious metal, it is used as a currency when trading on over-the-counter, exchange markets. In many countries, you can open a “metal” bank account in which this metal will be stored.
Palladium ring (Photo: Instagram / asyaair)
Alloys
Palladium is often mixed with other metals to change their physical or chemical properties. Additional components usually include:
- iridium;
- platinum;
- titanium;
- pure silver;
- cobalt;
- copper.
Up to 11% of this metal is contained in white gold. It is needed to give the latter a light silver tint.
Palladium jewelry is gradually gaining popularity, but this trend is not the most relevant. He has been known to the scientific community for many decades in other fields of activity.