Refining is the purification of metal from impurities.
This process consists of a series of sequential processes for separating excess components by physical and chemical means.
Some of the refining methods used in industry can be implemented at home, but sometimes the costs of carrying out the reactions exceed the profit from the resulting noble metal.
In this article we will tell you how to remove and separate gold from radio components and get it out of microcircuits, how to do it safely with your own hands.
Where is this precious metal found?
Hundreds of tons of gold are used annually in the production of radio components and computer chips. Contacts made of this metal are characterized by high electrical conductivity, they are not subject to oxidation, and therefore are widely used.

is contained in the following components :
- diodes;
- transistors;
- glass electrodes;
- relay;
- ports;
- jumpers;
- motherboard memory modules.
Note that in practice there may be much less gold in radio components than there should be according to documents (especially in technical products made after 1989).
Methods for removing impurities
The main method of isolating pure metal from mixtures, including from various radio components, is chemical refining . It is very common to dissolve it in aqua regia (a mixture of nitric and hydrochloric acid), followed by passing through a filter and reduction.
Electrolysis method
With the electrolysis method, gold from radio components or any other gold exposed to sulfuric or hydrochloric acid is deposited on the cathode when an electric current passes through the solution.
In industry, a cathode made of already purified gold is used; at home, you can use iron or lead .
A drop in current is a signal that the dissolution process is complete. This method is also effective and therefore quite common.
Cleansing with iodine
To etch gold from the surface of radio components, the most common pharmaceutical Lugol's solution is used - it is a mixture of iodine and potassium iodide. During the reaction, complex anions containing gold molecules are formed.
To increase speed, chemists add sulfuric or nitric acid . The dissolution process can continue for days. Subsequently, the noble metal is precipitated from the solution in different ways.
Using bleach "Whiteness"

Popular household bleach consists primarily of sodium hypochloride.
This substance, when mixed with hydrochloric acid, produces chlorine , which is subsequently used to dissolve gold to form gold chloride.
After this, sodium bisulfate is added .
At the end of the reaction, gray particles remain at the bottom of the vessel - this is gold, which will acquire a natural color after remelting.
Another option is to mix White, table salt (sodium chloride) and battery electrolyte, which is nothing more than sulfuric acid. The hypochlorous acid obtained during the reaction dissolves gold - it must be reduced in the future .
Refining "without acid"
Recipes spread on the Internet for obtaining gold from radio components and dissolving gold “without acid” essentially mislead readers, since acid (usually hydrochloric acid) is formed as a result of the reaction of other substances .
In addition, not everyone knows that the battery electrolyte used in such cases is also an acid.
Using Hydrogen Peroxide
The extraction of gold from radio components using hydrogen peroxide is carried out as follows.
This substance, otherwise called perhydrol, reacts with hydrochloric acid, dissolving gold. To do this, gold-containing raw materials are poured with acid and peroxide is added.
The resulting chloroauric acid is further decomposed into elements .
To do this, you can use a thermal method (point a blue flame of a burner at the substance) or a chemical one. The latter consists of reducing gold by adding ferrous sulfate.
Other extraction methods
There are many other refining methods that can be used to collect gold from chips, such as electrolyte and ammonium nitrate.
In this case, the electrolyte is mixed with ammonium nitrate - the so-called salt of nitric acid. The resulting composition is capable of dissolving the noble metal.
Most other methods also based on the dissolution of gold and its subsequent reduction .
The processes differ according to:
- cost;
- availability of components;
- reaction speed.
How to dissolve gold?
The process of extracting pure gold through its dissolution can be carried out in various ways. Some obtain it by cyanidation.
Cyanidation refers to the extraction procedure using potassium cyanide. Others use fluorine for separation.
It should be noted that this procedure is very dangerous. The fact is that the process is feasible only at extremely high temperatures. In addition, fluorine is one of the most toxic substances in nature.
The most popular method is to dissolve gold using aqua regia. It is noteworthy that “royal vodka” has been used in this area since 1270, and in Russia, since 1742, and this technique is still the undisputed leader.
Also, if there is a need to make 999 from ordinary 585 gold, “aqua regia” will prove to be an indispensable assistant here too.
Step-by-step instructions for extracting metal from radio components and microcircuits
To extract gold from microcircuits and radio components, it is advisable to use aqua regia .

To refine gold using this method, you must perform the following steps :
- grind the components mechanically, separating the parts that contain gold;
- get rid of organic matter by burning or calcining;
- open the windows in the room for better ventilation;
- for experiments, prepare a vessel made of borosilicate glass;
- place the workpieces in a concentrated mixture of 36% hydrochloric acid (3 parts) and 95% nitric acid (1 part) in small portions - up to 3 grams at a time, 500 ml of aqua regia will be required per 100 g of raw materials;
- Heat the solution while gradually adding nitric acid;
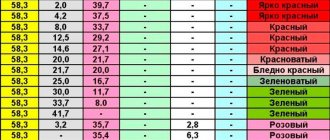
- check the presence of gold with tin chloride;
- filter the solution, then remove nitric acid from it.
Gold can subsequently be reduced using ferrous sulfate, perhydrol, oxalic acid or hydrazine sulfate. The resulting gold is smelted into an ingot using a crucible .
Note that it is unsafe to purify gold at home due to the significant causticity and toxicity of the substances used and the volatile compounds released.
In addition, advanced knowledge of chemistry will be required.
What is aqua regia?
Dissolving gold is a complex and time-consuming process; for this reason, alchemists have been trying to find a universal solvent for several centuries. They needed it not only to identify metal, but also to transform iron into gold.

Dissolving gold in aqua regia
The first descriptions of aqua regia appeared long before hydrochloric acid was discovered. Through trial and error, Pseudo-Geber obtained a mixture that, in his opinion, could dissolve any metal, including gold. This happened in Europe. The reaction took place using the following components:
- saltpeter;
- copper sulfate;
- ammonia;
- quartz.
The process of obtaining the solvent was carried out by dry distillation. The alchemist recommended preparing the mixture in a glass container that was tightly sealed.
The scientist Albert the Great called secondary vodka a mixture of nitric and hydrochloric acid. He considered nitric acid to be the primary vodka.
Bonaventura, the third researcher, described the mixture of acids as a solvent, he outlined his experiments on paper, and called the solution he was able to obtain “strong vodka.”
Scientists from Tsarist Russia also had an interest in chemistry; one of the first to describe in their works a mixture of hydrochloric and nitric acids was Mikhail Lomonosov. It is also noteworthy that initially the word “vodka” had no connection with strong alcoholic drinks. It came from the word water, only in the diminutive form. Gold does not dissolve in water - everyone knows this, but a mixture of acids has the transparency that is characteristic of water, for this reason it was called vodka.
When gold begins to dissolve or the solution interacts with air, the reaction occurs with the release of gas. Previously it was believed that these were vapors of the precious metal that evaporated during the reaction, but over the years it became known that the gas that is released during the dissolution of gold is chlorine.
Properties of aqua regia:
- dissolves gold and platinum group metals, provided that oxygen is involved in the reaction;
- used in the process of refining precious metals;
- the mixture of acids is transparent, but over time the solution gradually acquires an orange tint and loses its properties.
Gold dissolves in solution at room temperature, but if there is a need to speed up the reaction, the acid mixture is heated.
Several other properties of aqua regia can be noted:
- Does not dissolve silver (the metal forms a film).
- Does not dissolve Teflon.
- Zirconium, chromium, titanium and other elements are sensitive to solution.
Describing the properties of aqua regia, we can recall one interesting fact when German scientists managed to keep their awards.
Receiving the Nobel Prize in Germany was prohibited during the reign of Adolf Hitler. Due to the fact that the German chemist and opponent of the National Socialist Party, Karl von Ossietzky, once received the award.
Two German physicists, fearing confiscation, gave their awards to the chemist György de Hevesy. He hid the medals, but when the Germans occupied Copenhagen, the chemist was afraid that the awards would be confiscated. He dissolved the medals in aqua regia and placed the jar on the shelf. While searching the premises, the German military did not pay attention to the solution.
After the end of the war, the chemist restored the gold and sent it along with a letter to the Royal Swedish Academy, this happened three years later. The Nobel Foundation made new medals from gold and returned them to their owners.
Where to deliver the received material and at what price?
The gold ingot (barrel) obtained as a result of purification most often has insignificant weight. However, even in such quantities it is of interest to buyers.

Often, solid metal is purchased by the same companies that buy radio components.
(for example, aluminum or brass) may also be interested in gold
If the buyer is in the same city as the seller, the transaction is completed upon visiting the specified address.
The buyer himself weighs the metal and checks its quality, after which he sets a price. Of course, if possible, it is advisable to check all offers on the market in order to choose the most profitable one. The bullion can be sent to another city by mail, cash on delivery.
Some pawnshops are also ready to accept goods of this kind. To find out at what price the establishment will be willing to purchase gold, contact a pawnshop employee. Another option is to submit an ad yourself . In this case, you just have to wait for a call from a potential buyer.
If you engage in refining on an industrial scale, you should rely on existing laws.
To legally engage in the circulation of precious metals, you need to register as an individual entrepreneur or create an LLC.
When selling gold, focus on its purity and market value.
The price per gram of 999 fine precious metal is set by the Central Bank of Russia . Since 2004, its prices have been continuously rising. The most significant jump was observed in 2021, when the cost exceeded 3 thousand rubles per gram.