Alchemists in search of the philosopher's stone
In the middle of the last century, the news spread throughout the world that scientists had managed to synthesize gold artificially. Many took this news as the long-awaited news of confirmation of the receipt of the philosopher's stone. But not everything is as simple as we would like. The resulting gold had nothing to do with the philosopher's stone.
It is no secret that many alchemists of the Middle Ages searched for the philosopher's stone only in order to somehow convince their patrons to allocate funds for their chemical experiments and the study of occult sciences. As a result, humanity has gained a wealth of knowledge about the properties of chemicals. Over time, occult knowledge was forgotten and only some information that modern astrologers use has reached our time.
Later, scientists began to study the atom. They could be compared to modern alchemists, in the good sense of the word. They, just like their predecessors, walked at random, sometimes exposing their lives to mortal danger. And they also discovered unknown horizons of the structure of matter.
Deadly Friend - Quicksilver
The story of Nicolas Flamel
The story of Nicolas Flamel, a book copyist from Paris, is still considered mysterious. This man tried for a long time to get gold from mercury. There is a legend that back in the 14th century he unraveled a mystery that had interested people for centuries: is it possible to artificially produce a precious metal. It all started with the fact that an ancient manuscript with incomprehensible signs and symbols fell into the hands of this man. Nicolas tried to decipher this text for 20 years, but all efforts were unsuccessful. None of the experts in ancient languages to whom Flamel turned could help him.
To unravel the mystery of the manuscript, I had to travel outside of France. And only in Spain, after two years of searching for the right person, did luck smile on him. Here he met a real expert in the ancient Jewish language. The scientist, having learned about the manuscript, immediately went with Nicolas to Paris, since the copyist did not risk taking the texts with him. But the scientist was unable to reach France; on the way he fell ill and died. But still, he managed to tell Flamel something.

Armed with the knowledge gained, Flamel began deciphering the manuscript. His labors were not in vain; in January 1382, Nicolas was able to obtain silver from mercury, and soon his experiments with gold were crowned with success. Perhaps this is just a legend. But the fact is certain that the modest scribe became the owner of a huge fortune in a short period of time. After his death, many seekers searched his house for gold, but no one was able to find anything. There is still no evidence that Flamel knew how to make gold from mercury.
Unknown isotopes
While studying gold isotopes, American physicist Arthur Dempster discovered in 1935 that the noble metal has only one stable isotope with a relative mass of 197 . It is generally accepted that in order to synthesize it, one must have at one’s disposal an isotope with a much larger mass, but this simply does not exist in nature, and if it is synthesized artificially, then it cannot remain in a stable state for a long time. Therefore, all the efforts of scientists of the last century were aimed at obtaining a heavy isotope of gold.

Gold
This could only be achieved by using the elements closest to gold, mercury and platinum. It makes no sense to turn platinum into gold, since it is more expensive than it. What remains is mercury. In the early forties of the last century, research in this direction began in many nuclear laboratories. And in the spring of 1940 , physicists from Harvard University A. Scherr and K.T. Bainbridge was informed that they obtained the gold artificially. They managed to direct accelerated deuterons to a target made of lithium and thus obtain a flux of fast neutrons. In turn, the neutrons from the resulting lithium were used to bombard mercury. After conducting research, they came to the conclusion that gold was produced as a result of nuclear reactions.
But this gold consisted of unstable isotopes with mass numbers of 198 , 199 and 200 . After a few hours or days, it turned back into mercury, emitting beta rays into space. The reaction proceeds according to a well-known formula, which clearly describes this process well.
198Hg + n =198Au + p
Mercury has seven isotopes. And only three of them were able to turn into gold. Their mass numbers completely coincide with the numbers of the resulting gold. Later in March 1947, three physicists, colleagues of Professor Dempster Ingram, Hess and Gaidi, expressed a hypothesis, and after it proved that only 199 and 196 isotopes of mercury are capable of turning into gold. As a result of the experiment, they were able to obtain 35 100 grams of mercury . This reaction can be represented using the formula:
196Hg + n = 197Hg* + γ
But the process does not end there and continues further:
197Hg* + e- = 197Au

Mercury
How gold is made
One more example
Many years have passed since the discoveries of Nicolas Flamel. But the question of how to get gold from mercury remained open. Only at the end of the 19th century, the chemist Stefan Emmens announced to the whole world that he had managed to obtain a substance that could be called a precious metal.
The chemist called the substance obtained experimentally “argentaurum”, and it was made from silver, with the participation of mercury. Researchers from the USA carefully tested that substance and bought it back at the price of gold. These were three test bars. The scientist himself stated at the time that he was not going to disclose the technology and put gold into mass production, as this could have a bad effect on the economy not only of the United States, but of the whole world. But still, Emmens agreed to demonstrate the experience in Paris, at the World Exhibition. Shortly before the performance, the chemist disappeared without a trace. Most likely, his discovery was considered too dangerous.

Thus, gold was first obtained from mercury in laboratory conditions.
At first, no one attached any importance to this event. This fact was known only to those scientists who dealt with this problem. However, two years later, a certain meticulous journalist published the result of this research, providing the material with his own assumptions and reasoning. As a result, real panic began on the stock exchanges. Everyone thought that now gold would fall in price and cease to be a currency equivalent.
But there was no reason for the collapse of the stock exchanges. The resulting gold was many times more expensive than natural gold extracted from the poorest ores in a mine or gold mine. However, the physicists could not resist and allowed themselves a little luxury. Now a small amount of gold obtained in a nuclear reactor is stored in the Chicago Museum of Science and Industry, and in 1955 everyone could look at it during the Geneva Conference.
The secret is revealed
Now we will finally reveal the secret of obtaining gold from the “philosopher’s” stone. Naturally, this has nothing to do with alchemy. Everything we will operate with relates purely to the material world. And so, let's begin our reasoning.
To obtain gold from other chemical elements, it is necessary to take into account atomic reactions. To date, scientists have not discovered any other ways, so everything related to alchemy is considered erroneous, and their recipes are considered to be deception.
To obtain real gold, and not its isotopes, which do not last long, scientists, according to the nuclide map, considered several options.
- The first option
is when gold could be obtained from mercury-197 during beta ray emission or K-capture. But this is impossible in principle, because the 197th isotope simply does not exist in nature. Theoretically speaking, it can be obtained from thallium-197, and it, in turn, from lead-197. But such lead is formed only during nuclear reactions, and, unfortunately, it does not exist in nature either. So you won’t get much gold from simple lead. - The second option
involves the use of isotopes of platinum and mercury, which can only be formed during nuclear transformations. Therefore, gold can actually be obtained only from 196 and 198Hg and from 194 Pt. During bombardment with accelerated neutrons or alpha particles, reactions occur as a result of which isotopes of 197 Hg can be obtained, and from them, as is known, stable gold. But it will then need to be purified from the remaining isotopes that did not react and mixtures of various nuclides. And this is a very expensive cleaning method. Platinum as a source of gold can also be excluded for material reasons. - The third option
involves bombarding mercury with neutrons for a long time or using a cyclotron, but the yield of the substance will be very small. If natural mercury is irradiated with a neutron flux, then, as we have seen, radioactive isotopes are formed along with stable gold. After some time, they turn back into mercury and nothing can be done about it. This is how nature works.
Much more interesting is the process of obtaining platinum from mercury. It can be assumed that if powerful neutron radiation is directed in a reactor in such a way that (n, α) transformations occur, then one can hope to obtain a significant amount of platinum and all the isotopes of mercury that could be converted into gold.
What happened in the beginning
The most interesting thing is that the question of converting other elements into gold has always been before scientists. Even at the dawn of the study of the atom, Frederick Soddy suggested 1913 But then much was still unknown, and the reaction to which the scientist refers, for objective reasons, simply could not be carried out in an experimental setup.
Later, in 1938 , the science fiction writer Dauman proposed in one of his works a recipe for how to obtain gold from bismuth. He described how his hero, using powerful X-ray radiation, obtained gold in unlimited quantities from a piece of this substance. And then, using the method of literary conjecture, he modeled the political situation and analyzed it. Serious scientists immediately began to study the possibility of producing gold from bismuth, but quickly came to the conclusion that such a reaction was impossible because there is no stable isotope 205Bi in nature. The transformation formula can take the form
205Bi + γ = 197Au + 2α
What would happen if there was
Therefore, the hero of the novel could not have received gold. But we can take a risk and try to hypothetically imagine how, under industrial conditions, people will begin to obtain the noble metal from mercury. Based on knowledge from nuclear physics, we will begin our reasoning by using 50 kg of mercury. This amount of substance contains only 74 g of mercury-196, which can theoretically turn into gold.
Suppose from 74 g as a result of nuclear transformations we obtain the same amount of stable gold. After simple calculations, we come to the disappointing conclusion that 74 g of gold can be obtained by placing a ball of mercury with a capacity of 3.7 liters in the reactor zone for four and a half years. And then everything we get will need to be further cleaned.
As we see, it is simply not realistic to implement this in practice, but it is tempting. It is much easier and cheaper to obtain radioactive gold. It would be interesting to pay them, and then watch how over time it begins to melt and turn into mercury. Probably, in the future, scammers will learn to use this method, or it will simply remain on the pages of science fiction novels, constantly exciting inquisitive minds.
We turn everything upside down
Arguing about how gold can be obtained from mercury, we came to the conclusion that mercury can also be obtained from it. It turns out to be an interesting picture. It turns out that gold exists, most likely, contrary to the laws of nature. But reality is reality.
Currently, intensive work is underway to convert gold into other elements. If alchemists had known about this in their time, they certainly would not have understood us, their descendants. But a fact is a fact.
Scientists' research related to gold was not in vain. The fact is that at one time science was faced with the task of obtaining very pure mercury. No matter how they tried to purify natural mercury, nothing worked. That’s when they remembered that there is a reverse process, the transformation of gold into mercury. I had to start the reactor, suppressing my “greed”. This was done in order to obtain a very accurate meter standard.
Not all that glitters is gold
The first mercury vapor lamps appeared in the United States after World War II. As you may have guessed, the mercury in these lamps was artificial. Then the production of pure mercury was mastered in other countries. Radioactive gold-198 has also found application. It began to be used in medicine to treat cancer tumors and obtain radiographs of the human body. It turns out that tiny particles of radioactive gold kill cancer cells, leaving healthy ones unchanged. This method works locally without damaging a large surface. This method is recognized throughout the world and is preferred in many clinics.
Artificially produced gold is used to treat leukemia. It is known that during this disease the number of white blood cells increases. This method has saved the lives of many people suffering from seemingly incurable diseases. So humanity began to receive visible benefits from the use of a noble metal, albeit not durable and not so familiar, but nevertheless.
The interest of science in obtaining the “philosopher’s” stone fell. Now many laboratories are studying new substances that are synthesized from gold. The artificial elements francium and astatine are of great interest to scientists. Francium is produced by bombarding gold with oxygen or neon ions. Astatine is produced when gold is bombarded with accelerated carbon nuclei.
But it's not over yet
It would seem that we can put an end to this point. But how difficult it is to come to terms with the idea that it is impossible to obtain cheap gold from mercury. And it turns out that there are people who sincerely believe that this is not so. These are modern alchemists. Yes, they continue to develop this direction of research into the knowledge of the world.
What do we generally know about alchemy and the people who practiced it? History presents us with this direction in the form of fragments, telling us about successful experiments and unsuccessful experiences. There were probably many charlatans among the alchemists, but where are they not? Here is one example of how a fairly famous alchemist describes the production of gold from mercury. It looks something like this.
- You need to take the required amount of mercury and pour it into a vessel known to you. Then put it on the fire and boil the mercury for as long as you know. Throw a powder known only to you into the vessel. The quantity was told to you earlier. Thus, mercury will be fixed;
- Take a small piece of the resulting substance and throw it into a thousand ounces of mercury. It will turn into a red powder. Now throw a small quantity of this powder into a thousand ounces of mercury, and it too will turn into a red powder. Keep doing this until the mercury finally turns into gold.
Well, there is an “exact recipe” and reason for reflection. In any case, someone will someday use this recipe and, who knows, what new discoveries he will make.
What is mercury
Mercury is called "living silver". This silver-colored metal remains in a liquid state at temperatures down to -39 ° C and at the same time has extraordinary mobility. At temperatures below -39 ° C it becomes a solid metal.
Mercury is odorless and tasteless, and evaporates easily at room temperature. The vapors of this substance are very dangerous to human health. Therefore, in domestic conditions, a broken thermometer can cause severe poisoning.
Pure mercury is extracted from an ore called cinnabar. This mineral substance is specially heated to high temperatures so that the mercury can evaporate, and then it is condensed. The densities of mercury and gold are 13,600 kg/m3 and 19,300 kg/m3, respectively.
Liquid mercury has the ability to roll into a ball, and it also has a pronounced ability to wet certain metals. A mercury ball can attract gold dust and absorb it into its mass. Ultimately, when the ball can no longer absorb gold particles, it will begin to crumble as a single mass.
Amalgamation
Details Category:
AMALGAMATION
, the process of extracting gold (in some cases platinum and silver) from crushed ores or sands by selectively wetting metal particles with mercury. Waste rock particles are not wetted by mercury and do not transform into amalgam. In the first stage of extraction (wetting gold particles with mercury), the condition of the surface of the extracted metal particle is of great importance: deformation of the surface (crumpling, punctures, tears) and its rubbing (pressing in mineral dust particles) cause a significant decrease in extraction. In view of this, and also to avoid overgrinding, all modern amalgamation devices (sluices, amalgamators) are included in the grinding cycle to extract metal from recycled sand or from the pulp leaving the mill. In addition, a passivating oxide film is formed on the surface of metals, which can be removed by activation during the release of hydrogen (active amalgamation).
The capture of metal particles by mercury also largely depends on the properties of the latter, or rather, on the properties of the liquid phase of the amalgam, into which the initially pure mercury used for amalgamation quickly turns. The role of impurities contained in the liquid phase of the amalgam becomes clearer from the point of view of the modern theory of wetting and electrocapillary phenomena.
The features of the amalgamation process, which consist in the introduction of a number of components into the composition of the liquid phase of the amalgam, are explained by a decrease in the surface tension of mercury. As shown by the work of Plaksin and Kozhukhova, the conditions for amalgamation can easily be achieved. are represented by the cosine value of the contact angle of mercury wetting. The presence of a certain amount of noble metals and a small (0.1%) amount of heavy metals in mercury improves the wetting of the surface of gold particles due to a decrease in the surface tension of mercury and an increase in the cosine of the contact angle. In addition, it is worth noting Lipman's research, which showed that polarization of the mercury surface reduces its surface tension, and the maximum of the electrocapillary curve is obtained at zero potential of the mercury surface. Applying a negative pole to mercury (or gold) increases the cosine of the contact angle and accelerates wetting. The work of Academician A. Frumkin indicates a shift in the maximum of the electrocapillary curve for the phase represented in the case under consideration by mercury. In connection with the above, it is necessary to note long-known practical observations: 1) chemically pure mercury amalgams less easily than mercury containing gold and silver; in this direction are influenced by: a) a small amount of noble metals dissolved in mercury, which determines Ch. arr. wetting conditions and, to a lesser extent, diffusion of mercury to the center of the particle; b) the density of distribution of metal particles in the liquid phase, creating conditions for the absorption of particles wetted by mercury (above a certain limit it reduces the absorption of metal particles by the amalgam); 2) very small amounts of zinc, copper and lead contribute (according to Aur’s work) to amalgamation. At contents above 0.1%, these metals make mercury unsuitable for amalgamation. Gold and mercury form a solid solution (up to 16% Hg) and two chemical compounds. Stenbeek's latest X-ray study indicates the existence of six phases at ordinary temperatures, but the absence of X-ray studies at high temperatures makes it impossible to identify some of them with the phases established for the system diagram given by Plaksin's study. The solubility of gold in mercury at ordinary amalgamation temperatures (10-25°C) is in the range of 0.15-0.17% Au.
In terms of their structure, amalgams obtained during the extraction of gold in factories are polydisperse systems in which the solid phase consists of gold particles impregnated with mercury or surface amalgamated. The liquid phase consists of mercury containing a small amount of dissolved gold or other metal. That. The idea that gold is “dissolved” during the process of amalgamation extraction should be categorically rejected. Mercury, saturated with gold in a very low concentration, is returned to production after the extraction process. The fact that the solid (or plastic) part of the amalgam remains during the squeezing process on the filter clearly proves that it is not dissolved in an excess amount of mercury, but together with the latter forms a heterogeneous system with two phases: 1) the solid or plastic part of the amalgam, which represents a chemical compound (AuHg2) entirely or in the peripheral layer of particles, and 2) an excess amount of liquid mercury, in which gold is dissolved in a very low concentration. The structure of silver amalgams is in many ways similar to gold amalgams, but the conditions for trapping it during amalgamation differ from those for gold due to the slower wetting of silver with mercury. Amalgamation, based on trapping metal grains by wetting, is almost never used to extract silver. A number of processes previously used to extract silver were carried out either in vats with grinders or in rotating barrels in the presence of special additions of chemical reagents. Selective extraction of gold with a small amount of silver under normal amalgamation conditions indicates the connection of the latter with two categories of mineral inclusions: the first includes particles of silvery gold, and the second includes particles of native or golden silver and its chemical compounds (silver luster, horny silver, etc. ). According to the research of Tamman and Stassfurth, silver forms one chemical compound with mercury, corresponding to the formula Ag3Hg4. The quantitative composition of the solid phase of amalgams obtained in factory practice largely depends on the particle size of the amalgamated metal; Usually the ratio of gold to mercury is close to 1:2; Meanwhile, in the case of small gold, there is more mercury, and in the case of large gold, less.
The extraction of platinum by amalgamation is based on wetting its surface with mercury after preliminary preparation by exposure to chemical reagents. When amalgamating with zinc amalgam in a solution of sulfuric acid, the main reaction is the reduction of hydrogen on the surface of platinum particles, activating it and removing the film of adsorbed oxygen and oxides. In the case of ferrous platinum, it is advisable to pre-treat it with a weak (0.5%) solution of sulfuric acid to remove films of iron oxides. Along with the action of hydrogen on the surface of the particles, in the presence of copper sulfate, a film of freshly reduced copper is formed. As a result of the activation of hydrogen and the reduction of copper, the surface of platinum particles is easily wetted by mercury. Another method of amalgamation is pre-treatment of crushed ore or concentrate with a solution of chlorine and hydrochloric acid, followed by amalgamation with zinc amalgam. Along with the activation of the surface of particles by hydrogen, the formation of a film of platinum chloride PtCl2 is possible, which is slightly soluble in water, and upon reduction gives a layer that is easily wetted by mercury.
The transition to an amalgam of copper and iron is possible under abnormal conditions of amalgamation of gold ores, when the water entering the amalgamation apparatus contains soluble copper salts. When the ions of the latter are reduced to metallic copper, a copper amalgam is formed, into which particles of crushed iron are entrained. This process does not occur in alkaline water.
Source: Martens. Technical encyclopedia. Add. volume - 1936
- < Back
- Forward >
Amalgamation method
This method of extracting gold from mercury is considered one of the most ancient. It is very harmful to health, therefore it is banned in the Russian Federation, but in many countries it is still used.
Amalgamation is the process of mixing mercury and a metal such as gold. Mercury balls do not dissolve the metal, but only moisten it, absorbing it. Subsequently, using various methods, such as evaporation, pure gold is obtained.
This method is used if washing black sand and grains of gold smaller than one millimeter does not help.
Flushing
This method refers to the manual mining method, has been used since ancient times and has become widespread due to the high density of gold. Minerals with a lower density are simply washed away in a stream of water, resulting in the formation of a concentrate consisting of particles of sand and gold. If the volumes are small, a lazy washing tray is used; they are often used by prospectors to mine small placer deposits.
On a large scale, to obtain gold they use dredges (mining and processing units), mechanical equipment capable of working under water and, if necessary, excavating the bottom of the river. Industrial devices of varying power for gold mining are also a very popular mechanical device that is very popular among miners.
The resulting concentrates in this case may contain a number of other heavy metals, except gold. To remove them, other methods of obtaining gold are used, such as amalgamation.
Prohibited in Russia
In Russia, gold mining from mercury has been banned since 1988. At that time, the USSR Committee for Drugs and Metals issued an order “On stopping the use of mercury (amalgamation) in technological processes during the enrichment of gold ores and sands.” Before the publication of this document, the method using mercury was widespread in gold mining in the USSR. And the consumption of “liquid metal” in the gold mining industry reached hundreds of tons per year. At the same time, a huge amount of mercury entered the environment. To this day, gold miners find mercury waste in places where factories once stood.
Dangerous
Mercury vapor is very poisonous. Therefore, when working with this metal, it is necessary to observe safety precautions. Vapors should not be inhaled as this may cause serious poisoning. In addition, mercury and its compounds should not come into contact with the skin. When interacting with mercury, it is best to wear safety glasses and gloves, and the procedure for extracting gold using mercury should be performed in fresh air. In this case, it is advisable to make sure that the wind blows in the opposite direction from you and residential buildings.
Interaction with acid is as dangerous as interaction with mercury. For the reaction of gold and mercury, or rather to remove excess “liquid metal” during the amalgamation process, nitric acid is used. Therefore, you need to be especially careful when performing manipulations, take care of your skin and eyes, and avoid inhaling acid fumes. To wash off any acid that gets on your skin, you can use clean water.
There is one more rule: when making a solution, it is best to pour the acid into water, and not vice versa. This will help avoid splashing. You can neutralize the effect of acid using soda.
When working with acid, clean water should always be on hand to quickly dilute the acid in case of contact with skin or equipment.
Acid getting on the body causes burns if it is not washed off immediately. Even if it gets on clothing, it will most likely penetrate the skin. In this case, you need to take off your clothes and wash the burned area. It is also recommended to wear a special mask when working with acid; this will help prevent burning your lungs when inhaling the vapors.
Versions: Mercury gold of the Incas influenced the genes of Europeans
The fearless Spaniards who conquered America had no idea how dangerous the Incas' gold was. After all, ancient civilizations mined gold using mercury, which slowly but surely kills not only the owner of the treasure, but even his future offspring...
At the beginning of the 16th century, when, as a result of the Great Geographical Discoveries, the new continent of America appeared on the map, Europeans had no idea what this threatened them with. And during the first decades of the conquest of new lands by the conquistadors, Europe listened with delight to overseas gifts: potatoes, tobacco, coffee and the main wealth of the new colonies - gold.
From the very moment when Cortez's soldiers saw that the new land was literally overflowing with the noble metal, they thought of nothing else.
The Inca Golden Bird on display in Prague in 2008. Photo: wikimedia.org

Amazing architecture, deep knowledge of astronomy and mathematics, the ability to predict disasters, grow rich harvests and fight drought - all the rich knowledge of the Incas, Mayans and Aztecs was a free addition to the most important treasure of ancient civilizations. The “yellow devil” (as gold was called in Europe) clouded the eyes of the conquerors so much that they did not want to know anything else.
And no one thought about where these essentially wild peoples at a low stage of development got so much gold? After all, Europeans knew that mining precious metals was a complex technological process, and that smelting and manufacturing jewelry required considerable knowledge and skills. And the fact that the methods used in gold mining are not at all harmless.
Where does the metal come from?
Gold, for which the Inca Empire was famous, was considered a sacred metal - the metal of the Sun God. Gold lay in bulk in the temples; walls, floors and ceilings were decorated with it (the ceiling of one of the temples was strewn with openwork golden stars, golden dragonflies, butterflies, birds that soared above people and were so magnificent that their beauty awed everyone saw them).
Silver plates and gold cup of the Incas in the Latin American State Museum of Ethnology in Germany. Photo: Photo: wikimedia.org

In 1532, the Spanish conquistadors kidnapped the Inca emperor Atahualpa in order to take a ransom for him in the form of gold in an amount equal to the volume of the room in which the arrested emperor was kept. The Indians set off to fulfill the demands of the conquistadors. Caravans of mules and endless lines of people carried statues, gold jewelry, precious vessels and other items made of precious metals to the capital for a week. There was so much gold captured by the Spaniards that, after melting it into bars, the Spaniards carried it home in galleons.
It is estimated that in the entire history of mankind, only 161 thousand tons of gold have been mined in the world. But in the 16th century, Spanish galleons delivered more than 100 tons of gold to the court of the Spanish king in just one year (in Europe at that time no more than 1 ton of gold was mined per year).
Of course, such “rich times” existed only at the beginning, when the Spaniards destroyed the treasuries of the Aztecs, Incas, Mayans and other peoples. In total, the Spaniards exported more than a thousand tons of “poisonous” gold from South America.
But in order to have such a wealth of precious metal, ancient civilizations had to mine it on an industrial scale. And that's how they got it. But the conquistadors did not care about Incan technology; they needed to steal as much treasure as possible. And they didn’t want to know what it was - high-quality, low-quality or completely poisoned.
Meanwhile, as it turned out later, the Inca gold was produced with gross violations of technology: it was smelted using mercury. Consequently, all the gold brought to Europe was actually poisoned.
So the cruel conquistadors doomed not only the ancient civilizations of America to death, they also planted a time bomb under European civilization. Europe reaped the fruits of thoughtless greed and predation for several centuries. However, she reaps them to this day, which is visible to the naked eye.
But what is this method that, like an old woman with a scythe, mercilessly knocked down the descendants of the proud conquerors?
While there is life there is hope?
By the time of the Spanish conquest, the technology of gold smelting was well known in Europe - a similar method was used by the ancient Romans. It was as follows: to extract gold from ore, crushed rock was mixed with liquid mercury. In this case, the resulting alloy, or amalgam, was easily separated from the total mass as heavier. The amalgam was heated to a high temperature, the mercury evaporated, and as a result, pure gold remained.
Some believe that the Spaniards brought such technology to America. Indeed, in Spain at that time there was the largest deposit of mercury, and local gold miners were considered monopolists on the European continent. Therefore, no one was surprised that all the gold smelted before the period of the American conquest was mixed with mercury.
Photo: wikimedia.org

But there wasn’t very much of it, so no one attached much importance to this feature. But the gold products of the Aztecs, Mayans, Incas, smelted before the advent of Europeans, were always considered “pure” - everyone thought that they did not know mercury and smelted gold according to their ancient and, of course, harmless recipes.
But, as it turned out, nothing like that - recent studies have shown that ancient civilizations also used mercury, and no less intensively than Europeans. The same Incas, as it turned out, in addition to smelting gold, also used mercury to produce cinnabar, used as a dye.
Clothing dyed red-orange was considered the property of the rich and was the favorite clothing of the nobility. Addiction to this extremely dangerous and harmful substance, according to one version, became the reason for the degeneration of an entire people - after all, by the time the Spaniards arrived, the Incas were so weakened that the conquistadors conquered the continent with a small detachment and practically without casualties.
Revenge of the Gods
But the revenge of the ancient gods was just around the corner: after all, all the gold that came to the courts of European monarchs was mortally dangerous. Europe was actually covered by a golden (read “mercury”) wave - there was not a single royal court on the continent, not a single noble family that, to one degree or another, did not have American gold in their hands (and how could it be otherwise, if its quantity increased almost by 200 times?).
They ate on gold - plates, bowls, cups, dishes, forks, spoons; they slept on gold - beds, armchairs, chairs, thrones; they wore gold - crosses, jewelry, crowns, scepters, rings, chains.
The Ugly Duchess, 1525-1530 National Gallery, London. Photo: wikimedia.org

And it is not surprising that most European royal courts were ultimately doomed to degeneration - there was not a single crowned family that did without serious diseases passed on from generation to generation.
And for all this we can “thank” the Incas, who not only doomed themselves to death, but also passed the “relay of death” to European civilization.
Or maybe this was a sophisticated revenge of a people who had disappeared into oblivion? Author: Egor SHVARTS
Preparation
In order for mercury to absorb gold, it is necessary to clean it of foreign impurities, since they will interfere with the amalgamation process. Sometimes the precious metal is coated with a film of oil or other impurities. A ten percent nitric acid solution is used for cleaning. The washed concentrate is poured into it.
During this process, a reaction may occur that releases gas. It is necessary to wait until all signs of the reaction cease and then rinse the concentrate with clean water, thus washing off the acid.

The process itself
The entire process is carried out in a steel or plastic rinsing tray. The amount of mercury must be equal to the amount of gold in the concentrate. You don’t need too much mercury, since in this case it will be inconvenient to work with. It is better to initially pour less and gradually add more. There should also be a small amount of water in the tray during the process:
- We take the tray in our hands and make circular movements until all the gold that was visible is combined with a ball of mercury. Black sand does not absorb mercury.
- After this, wash off the black sand into a basin of water.
- If during this process a small amount of amalgam leaks into the basin, do not worry. It can always be easily removed from a basin of water.
- We mean that mercury does not capture platinum. Therefore, we carefully watch during the final rinsing.
- If during the process the mercury ball begins to separate, add a little more mercury so that all the gold contained in the sand is absorbed.
- A mercury bead that is completely filled with gold will be 50% mercury and 50% precious metal.

How to make gold from mercury
Once all the gold has been amalgamated and the amalgam itself has been separated from the sand, you can begin to remove the excess mercury. This can be done using extrusion. To do this, take thin and damp suede or any other dense material. All excess mercury must pass through the pores of the fabric. It is best to fill the container that will be located under the material with water. This way, excess mercury will not be splashed, and it can be easily collected in the future. This process is best done while wearing rubber gloves to prevent the mercury from being absorbed into the skin.
The mercury that has been squeezed out of the amalgam will contain a small amount of gold. These residues will help collect more precious metal in future amalgamation processes.
Once all excess mercury compounds have been removed from the ball, you can begin to separate the gold from the mercury. To do this, you can use one of two methods: evaporation, by heating the amalgam, or dissolving mercury in nitric acid.
Cyanidation
Cyanidation is a hydrometalurgical chemical-technological process for extracting gold from siliceous and silver ores, based on the fact that gold and silver are highly soluble in cyanides, which include potassium cyanide and sodium cyanide. The reaction of gold with cyanide occurs in the presence of oxygen contained in the air; after obtaining the desired solution, the precious metal is precipitated with zinc metal. This method includes two stages, which have the formulas:
- Treatment of gold-bearing rock with sodium cyanide solution (less than 1%):
4Au + 8NaCN + O2+ 2H2O → 4Na[Au(CN)2] + 4NaOH; - Precipitation of gold from sodium cyanoaurate using zinc dust:
2Na[Au(CN)2] + Zn → Na2[Zn(CN)2] + 2Au↓.
Cyanidation becomes impossible if the ore contains large amounts of sulfides or arsenides, since cyanide reacts with these substances. flotation method is successfully used , when gold particles are separated from the ore mass using an aqueous solution. The technological process takes place in special flotation units and a phase separation formula is used, when solid particles of precious metals that are poorly wetted by water are separated from well-wetted sulfides.
Evaporation
Mercury evaporates at a temperature of 357 degrees, which is found in the upper parts of the flame of gas burners. Since mercury vapor is highly toxic and can cause fatal poisoning, this procedure should be carried out outdoors. In this case, the wind should not blow towards the person. Mercury can be present on gold in the form of a thin, invisible film, so if the metal appears clean, do not assume that there is no mercury on it.
You can use a steel tray or frying pan for this process. Aluminum containers are not very suitable for evaporation, as aluminum can react with mercury.
Before heating the amalgam bead in the tray, you need to remove as much mercury as possible from it using the method indicated above. At first, the ball is heated slowly, gradually increasing the temperature. If gold contains small amounts of mercury compounds, you don't have to worry about them spattering.
content .. 71 72Seawater amalgamation for gold recovery
The amalgamation process and equipment for extracting gold in metallic form from seawater were proposed as early as 1903.
Pre-filtered seawater was pumped through a tube to the bottom of a conical funnel-shaped vessel containing mercury and divided into many sections by perforated sheets (Fig. 92). Once brought into contact with the mercury, the upward flow of water was passed through a screen to catch the fine pumice mercury, then through perforated contact sheets, and finally through an amalgamation sluice located at the top of the apparatus and designed to completely capture the amalgamated gold from the flow. The amalgam was processed using generally accepted methods (squeezing, stripping and melting).
Similar equipment was proposed by Ritter1 and differs in that the thin mercury and the gold it contains, having passed through the mesh, are captured in a corrugated device.
Ion flotation
As noted above (see Chapter IV), ion flotation is based on the ability of some heteropolar compounds to interact with ions of heavy metals, and in particular gold, to form a flotable insoluble compound. The most famous work in this direction is in relation to the sea water of Sebba (South Africa) 189 J.
Sorption
Carbon-containing materials were tested as one of the first sorbents for extracting gold from sea water. Thus, at the beginning of the 20th century, Parker established that viscous carbon-containing materials such as asphalt, bitumen, mineral resin and others have an affinity for free gold. On this basis, Parker proposed capturing finely dispersed (or so-called floating) gold from sea water by selectively fixing it on solid viscous carbon-containing beds deposited on bars and strips installed in the flow. Ensuring continuous contact of fresh water with the viscous material should be carried out by the action of the ebb and flow of the sea [129].
However, most researchers believe that among the carbon-containing sorbents, activated carbons are the most interesting for the sorption of gold from sea water.
The pioneers of this direction were German researchers Nagel and Baur (1912-1913), who proposed using coke, charcoal and animal charcoal and some other adsorbents for the sorption of gold from sea water. In the experiments, seawater, after preliminary clarification using a sand filter (to remove suspended material and gelatinous microorganisms), was passed through a filter bed of coke, coal or other carbon-containing material using the method of free percolation or ascending filtration (Fig. 93). The enriched adsorbent was periodically removed and melted.
To reduce the cost of pumping seawater, it is proposed to use perforated containers with an adsorbent bed on board the ship, or coastal tanks with a false bottom and a layer of adsorbent covered with wire or fabric mesh, filled by the action of the tides.
In parallel with the use of a classic adsorbent (active carbons), studies were carried out with inorganic sorbents with a highly developed surface, such as freshly precipitated hydroxides (aluminum, iron, silica gel), coagulated hydrocellulose, etc. In this case, it was proposed to use coastal vats or special stands filled with inorganic sorbent and completely covered with a double layer of fibrous textile material. The stands are immersed in sea water for weeks, and often months, after which they are exposed to cyanide solutions to extract the adsorbed gold. Gold-plated stands are used repeatedly.
When investigating possible sorption methods, it was found that colloidal metallic gold is preferably recovered in this process. Therefore, it was natural to look for a sorbent that would simultaneously reduce halogen gold to a metallic state and create a freshly formed active surface. Having examined a wide range of such possible sorbents, Parker came to the conclusion that for the most complete extraction of gold from sea water, ferrous sulfate is preferable, the optimal consumption of which is 2 kg/t of water.
Subsequently, Parker received a separate patent2 for the hardware design of the adsorption method using ferrous sulfite.
The combination of the processes of halide reduction and adsorption of colloidal gold is also observed in the proposals of other researchers. Thus, Bardt recommended treating seawater with sulfite liquor (a waste product from cellulose production) as a reducing agent, followed by mixing it with a mixture of finely ground coal and atomized metal (for example, copper, iron, etc.) 3. The sediment containing noble metals was first burned ( to remove carbon) and then smelted, collecting gold in the accompanying metal.
A similar goal (reduction of halide gold and complete capture of colloidal gold) was pursued by Glazunov and his co-workers (Paris, 1928), proposing the use of sulfides, and in particular pyrites, as an adsorbent for gold dissolved in sea water [142].
This idea was practically realized only in 1953 by Walters and Stillman, who went their own original way. According to their proposal, the sulphide ore was piled behind a concrete wall built near the lower tide line and curved towards the shore. At high tide the ore was submerged by water, and at low tide the water percolated through the ore. This cycle was repeated many times. After a certain time, the decomposed sulfide sludge containing adsorbed gold was removed at low tide and smelted. The inventors noted that the precipitation of gold by sulfides is facilitated when seawater is exposed to radioactive elements.
Stokes later showed that a variety of natural and artificial sulfide materials can be used to precipitate gold from seawater, with antimony sulfide being very effective.
To intensify the process of gold sorption by sulfides, while simultaneously eliminating the cost of pumping sea water, Gernik and Stokes proposed a special apparatus called in the literature “antimony-sulfide trap” (since it was conceived for use as an adsorbent, antimony sulfide) or "tidal energy system". This apparatus is made in the form of an inverted U-shaped pipe, in one elbow of which there is an expansion into which an adsorbent (activated carbon or sulfides) is placed between the grids. Sea water flows through this tube under the influence of a tidal current or during the movement of a vessel to which the described apparatus is attached.
Over the past 10-15 years, a number of patents have appeared that improve the sorption extraction of gold from sea water using metal sulfides 2. The most original idea and equipment in this direction were presented by the American researcher Norris 3.
His latest invention is based on the use of freshly precipitated metal sulfide colloids adsorbed on the surface of durable organic, synthetic or natural fibers. A typical example of synthesized organic fibers is polymerized acrylonitrile or vinyl cyanide fibers. Of the natural fibers, the most suitable is Ramie fiber (Chinese nettle). Such fibers, if immersed in a thin colloidal suspension (for example, freshly precipitated zinc sulfide prepared by mixing dilute solutions of zinc chloride and sodium sulfide at a pH value of approximately 6.0), will actively adsorb a significant portion of the colloidal sulfide particles and firmly retain them on their surface .
When sorption fibers prepared in this way come into contact with poor gold-containing solutions (for example, sea water), noble metal ions are adsorbed. They can be removed from the fibers by treating with heated dilute solutions of sodium cyanide with a small addition of hydrogen peroxide or sodium hypochlorite with a small addition of hydrochloric acid. Once the adsorbed ions have eluted, the fibers can be washed and reused repeatedly after pretreatment with a zinc sulfide slurry. In addition to zinc sulfide, iron, manganese, copper, nickel and lead sulfides can be used in this process.
Long-term research by Norris has established that certain oxidizing gases, which are often dissolved in most sea waters, can adversely affect the collectors and adsorption fibers used. These gases include oxygen, nitrogen and carbon dioxide. Therefore, to achieve the greatest effect, the proposed apparatus must have a means of continuously removing such gases from the flowing seawater before it comes into contact with the collecting structure of the fibers. Moreover, due to the relatively small number of metal ions that are collected in one normal operation, as well as the complexity of processing and handling the fiber mass, it is advisable to perform all operations continuously and automatically. All these factors were taken into account in the apparatus proposed by Norris (Fig. 94).
Of particular interest to researchers is the use of natural and artificial ion exchangers to extract gold and silver from seawater.
Priority in this direction belongs to Brook, who in 1953 proposed using iron and manganese zeolites to extract silver from sea water
Later, in 1964, Bayer and his colleagues (Germany) created so-called chelate ion exchange resins, capable of extracting up to 100% of valuable metals from sea water.
Of the most recent works devoted to the use of solid ion exchangers for the extraction of gold from sea water, the most interesting is the study of a group of experimenters from the Guff Research and Development Company (USA).
To collect precious metals, it is proposed to use a water-insoluble ethylene polymer containing pendant carboxylate or amide groups. One of the best ways to obtain this polymer is the saponification of an ethylene alkyl acrylate copolymer or the synthesis of a copolymer of ethylene and an ester of acidic groups, including maleic, fumaric and taconic acids. The production of such sorbents is described in detail in the patent.
Upon reaching a sufficient degree of loading of the polymer film, sorbed gold can be extracted by smelting from ash after burning the polymer or precipitated from solutions from dissolving polymers in caustic soda (caustic soda).
The ways of using natural and artificial ion exchangers are basically the same as the sorbents discussed above, namely: installation in a stream of sea water, filtration through a bed in a vat, loading of porous containers.
Merro proposed a completely new way of using artificial ion exchangers [179] - applying them to the hull of a ship making its commercial voyage. Upon arrival at the destination port, the ion exchange resin can be stripped from the vessel and processed. Resin processing consists of washing with acids and special elements, followed by electrolysis of the eluate containing noble metals. Regenerated resins can be used repeatedly.
The most economical proposal is to use special devices located in the hold of the ship and filled with ion-exchange resins [180]. Here it is provided that the forward movement of the vessel causes sea water to continuously flow through the vessel with the ion exchanger. This vessel should have a cross-sectional area of about 9.5-10 m2, a length of 3 m and contain about 28 m3 of resin. The maximum flow rate of sea water during sorption onto the resin should be -0.8 m3 through 1 m2 of surface per minute (0.8 m/min).
At this flow rate, -12,500 tons of seawater will pass through the sorption device per day. Even when kept in water
1 mg!t of gold per day will yield 12.5 g of gold. During a year of continuous voyage, about 4.5 kg of gold, worth about $5,000, can be adsorbed.
Cementation
One of the few information about the practical application of the method of cementing gold from sea water relates to the Parker method patented in the USA. Nickel dust has been proposed as a cementitious metal. By reduction, substitution and adsorption, gold, present in both halogen and elemental forms, can be isolated from seawater.
When carrying out cementation by mixing nickel powder with sea water, it is possible to achieve a gold loading of 15 to 20% by weight. The loaded nickel powder is removed from the vat and melted.
To precipitate gold from very poor sea waters, Sneeming proposed using the increased affinity of gold for tellurium. It has been established that it is most advisable to carry out deposition with amorphous tellurium with a highly developed reaction surface. Such a cementitious agent is obtained by treating soluble tellurium salt with sulfur dioxide. Seawater is filtered through a fixed layer of amorphous tellurium. To extract the deposited gold, the enriched mass is heated to sublimate tellurium (with its subsequent capture), and the remainder is melted into gold.
Acid method
Nitric acid is often used to separate gold from mercury after the amalgamation process. By reacting with mercury, it dissolves it without having any effect on the gold. Before you begin, you need to make sure that the amalgam does not contain excess mercury and black sand impurities:
- Place the mercury ball in a glass jar.
- Pour in an acid solution in a ratio of 6:1, stronger if possible.
- We wait until the chemical reaction takes place.
- We rinse the jar well with clean water and pour it into a separate container.
- If the gold has not taken its natural form of flakes and powder and remains of mercury are visible, drain the water and pour in another portion of nitric acid. In case of another failure, we make a stronger solution.
As a rule, with a small amount of mercury, cleansing occurs the first time. If there is a lot of mercury, all steps will need to be completed several times.
If using this method a large amount of “liquid metal” is dissolved and there is a desire to preserve it, you can use the following method:
- Drain the acid after the process into a separate jar. It will contain mercury that has been removed from the amalgam.
- Place aluminum foil in the jar.
- The acid will react with the aluminum and precipitate mercury to the bottom of the jar.
- We drain the acid from the container, neutralizing it with baking soda, until the gas evolution stops.

Where gold comes from - you can find out very accurately
In many fields - from forensic science to art history - there is an urgent need to identify gold-containing materials. Recently, the methods of such identification and the efficiency of recognizing the “fingerprints” of a specific gold sample have made significant progress.
Here the term “fingerprinting” is used not in a narrow sense (a method of identifying a person by fingerprints from the Greek Daktilos - finger), but in a broad sense - like the terms “genetic fingerprinting” in biology, “steganographic fingerprinting” in computer science, “brain fingerprinting” in psychology. By the way, in the English-language literature the term Gold fingerprinting (GF) has been used for 20 years to identify the origin of a gold object.
Typical instruments for gold analysis
Read more
The main types of materials requiring fingerprinting, precise control and accounting are:
- gold-bearing minerals (primarily industrial ores, gold nuggets and placer gold sand);
- products and semi-products of gold mining factories (Dore alloy, etc.);
- refined gold (including bank and measured bars);
- jewelry (including jewelry solders);
- artistic gold (gold paints, gold foil, including for religious items);
- recycled gold (including scrap, jewelry and electrical scrap, recovered gold, dental waste, etc.).
Gold fingerprinting is considered as a complement and scientific alternative to descriptive certification and certification of gold materials - documents can be forged, as well as to the hallmarking of jewelry and gold bars - hallmarks can be interrupted or falsified.
Potential consumers of gold fingerprinting databases are law enforcement agencies (FSB, Ministry of Internal Affairs, customs service, courts and prosecutors, etc.), companies involved in gold trading and jewelry manufacturing, research centers, including archaeological ones, as well as the Gokhran of the Russian Federation, museums and art organizations.
It is difficult to reliably assess the current global level of scientific research in this area, since the results remain confidential due to fierce economic competition and even the influence of the black market, whose representatives are not interested in improving objective methods of monitoring gold materials.
Nuggets
In the field of raw materials, the greatest successes have been achieved in the fingerprinting of native gold. In indigenous sources, gold is primarily found in three forms:
- isolation of free native gold;
- gold compounds, mainly tellurides and much less frequently selenides, sulfides, antimonides;
- dispersed admixture of gold (submicron particles) in various minerals, mainly in sulfides.
Native gold is characterized by a wide variety of forms of isolation: vein-plate-like (to scaly), spongy, lump-like, branched, crystalline, drusen-like, dendritic, wire-like, needle-like, angular, drop-shaped. A common feature is the wider development of faceted forms among small gold deposits and irregular forms among large ones. Apparently, there is a certain dependence of the forms on the depth of formation of gold deposits. In particular, in shallow deposits, elongated, even hair-like and flattened, and dendritic gold deposits are more developed.
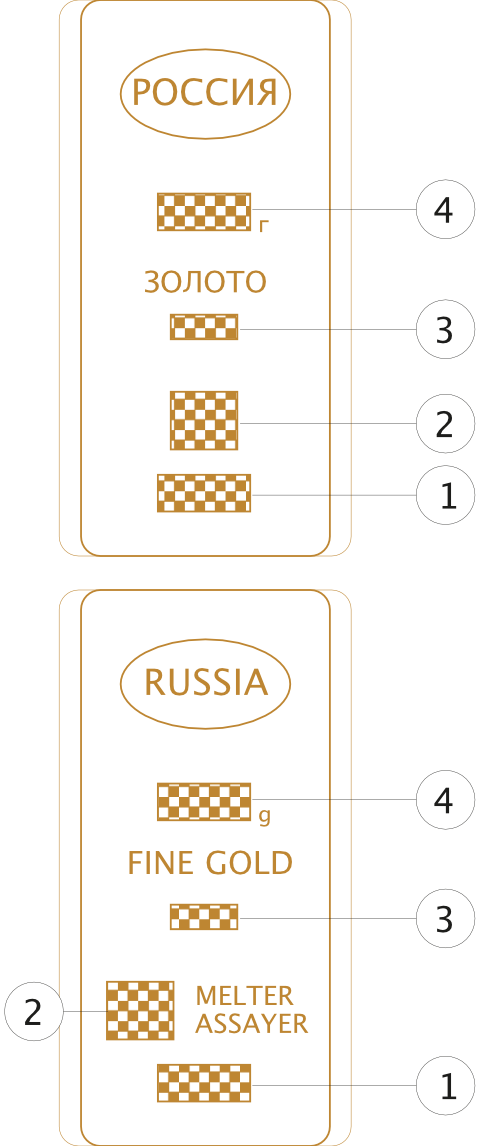
The order of marking of a measured gold bar in Russia
1 — number (code) of the ingot;
2 — manufacturer’s trademark;
3—mass fraction of gold in samples;
4 - nominal weight of the ingot
The permanent impurity elements in native gold are Ag, Hg, Cu, Pd, and less commonly Sb, Cd, Pt, which form limited solid solutions and intermetallic compounds with it. The distribution of these isomorphic impurities in gold is usually uniform, which distinguishes them from unevenly distributed mechanical impurities. For example, in the gold-bismuth deposits of Yakutia, localized both in the zone of endo- and exocontact of intrusions of large batholith belts (Tuguchak, Basagugunya), and in the zone of local granite massifs (Kurum, Dyby, Teutedzhak, etc.), as a rare but Maldonite is a permanent mineral. In most cases it is associated with bismuth tellurides, native gold and bismuth, and less commonly with bismuth sulfotellurides. A significant number of different compounds of gold with antimony are noted in gold-antimony deposits (Maltan, Sarylakh, Gan, El, Sentachan), which are controlled by the Adycha-Taryn fault, which has been repeatedly activated. This type of deposit is polygenic and is associated with the superposition of young antimony mineralization on early gold mineralization.
In Russia, a multi-stage gold fingerprinting technique was developed by the Central Scientific Research Geological Prospecting Institute of Non-Ferrous and Precious Metals. It has found application not only in geology, but also in forensic practice. After all, establishing the location of extraction of native gold seized from illegal circulation is one of the main tasks of the investigation. The answer to this question allows us to identify and block the sources of gold thefts, transportation routes for stolen metal, identify those involved in the illegal circulation of gold, etc. In recent years, the methodology for forensic research of native gold has been significantly modernized. As one of the authors of this methodology, Colonel of the FSB of Russia, Candidate of Geological and Mineralogical Sciences Georgy Samosorov, explained, at a certain stage it became obvious that the existing methodology was outdated and did not correspond to the modern level of development of science and technology, and the incompleteness of the existing data bank made it difficult to use it to obtain evidence information. “The modernization of the methodology consisted of an in-depth study of the geochemical characteristics of native gold. The scheme of its comprehensive study includes a wide range of modern precision analysis methods - scanning electron microscopy, X-ray spectral microanalysis, mass spectrometry with inductively coupled plasma, optical emission spectrometry with inductively coupled plasma and Auger electron spectroscopy. This made it possible to obtain fundamentally new data on the characteristics of gold from different types of deposits in Russia, and to create its most complete geochemical models,” said Georgy Samosorov.
Failures in fingerprinting works of art
In the early 2000s, methods for identifying pictorial layers using man-made isotopes of strontium-90 and cesium-137 were patented.
Testing was carried out in several centers, including St. Petersburg. Art dealers, auction houses, and experts put the brakes on the matter, because a large number of works of art with high insurance values are, as a result of repeated restorations, Frankensteins. The use of the modernized methodology makes it possible to obtain qualitatively new information from the results of studying native gold: to establish the place of its extraction, regardless of the amount of material, as well as the degree of its primary enrichment (this is, for example, amalgamation, mechanical deformation or alloying in artisanal conditions). Based on this methodology, a database of gold deposits in 11 largest gold-mining regions of Russia was created. It contains quantitative values of the characteristic features of gold for each deposit, values of gold fineness ranges determined by assay analysis and X-ray microanalysis, data on the geochemical characteristics of gold, etc.
The data bank can be expanded due to newly received materials. A specialized information retrieval system has been developed based on modern geoinformation technologies. It allows for an automated search for analogues available in the data bank by generating queries for each characteristic of gold. Reducing the list of objects is carried out by gradually clarifying the search conditions, which ultimately makes it possible to identify one or more objects with gold similar to that submitted for research. At the initial stage, metallogenic zones and ore areas are established based on a set of primary characteristics of gold (granulometry, fineness, impurity elements, morphological types, surface topography). In the absence of data on any of these characteristics, conclusions may be ambiguous. To clarify them, at the final stage, data on intergrowths with other minerals and films on the surface of gold grains, their internal structure, as well as morphogenetic types of gold are analyzed, which makes it possible to determine a specific deposit. If the native gold submitted for research has been subjected to any human influence (amalgamation, mechanical deformation, artificial sampling or mixed sample, etc.), then gold identification is carried out according to the preserved characteristics. In this case, the result will be less reliable.
Diamond fingerprinting
Read more
The accuracy of conclusions increases markedly when using inductively coupled plasma mass spectrometry data on the composition of trace elements in gold. Based on the processing of the results obtained, geochemical indicators were calculated for a number of regions, which were used as a new criterion in determining the source of origin of native gold. Using the methods of Auger spectroscopy, electron microscopy and X-ray microanalysis, new data were obtained indicating that on the surface of gold there are thin films of various compositions, micron and submicron mineral inclusions, and other compounds and phases. The composition of these formations and the nature of their distribution make it possible to distinguish gold not only from deposits of different ore formations and gold-bearing provinces, but from deposits of the same formation type within the same gold-bearing province.
Fingerprints of Africa and South America
The famous transnational corporation Anglo American has developed a commercial product - a database of gold fingerprints from various deposits in Africa and South America.
The main method is mass spectrometry with inductively coupled plasma and laser ablation (Laser Ablation Inductively Coupled Plasma Mass Spectrometry). 131 isotopes from Sc45 to U238 are recorded. The spectrum processing program is sold together with the database. The developers claim that a “fingerprint” follows the gold from the deposit to the refinery, so it is easy to determine from which deposit the gold was stolen. The method was successfully applied to the analysis of gold archaeological artifacts from the Mapungubwe kingdom, which existed in South Africa in 1075–1220. The technique is claimed to be applicable to platinum metals and other objects. Gold bars
Since January 1, 2021, GOST 28058-2015 “Gold bullion. Technical conditions". This standard, developed by specialists of the Interstate Technical Committee for Standardization MTK 304 “Precious Metals, Alloys and Industrial Products Made of Them” and JSC “Ekaterinburg Non-Ferrous Metals Processing Plant”, applies to refined gold bullion intended for the needs of the country and for export. Depending on the chemical composition, ingots are made from gold grades ZlA-1P, ZlA-1, ZlA-2, ZlA-3, ZlA-4. Gold bars should have the shape of a truncated pyramid, the bases of which are rectangles.
Ingots are manufactured weighing from 11,000.0 to 13,300.0 g. The total proportion of impurities can be up to 0.01%. The content of 17 microimpurities is a kind of “fingerprint” of each gold bar. In addition, it is possible to add special markers from rare metals, such as zirconium, hafnium, tungsten, molybdenum, etc., to the amount of microimpurities. Marker content numbers (for example, hafnium 0.002% + zirconium 0.001%) can encode individual information and thereby provide additional secret information — fingerprinting of gold bars.
For several years now, the Gokhran of the Russian Federation has been using the analysis of bank and measured gold bars in practice. The methods of atomic emission spectrometry with inductively coupled plasma and spark ablation and mass spectrometry with inductively coupled plasma and laser ablation are used to control the chemical composition of 28 impurity elements. A corresponding database is constantly maintained.
Vladimir Teslenko, Candidate of Chemical Sciences
Use of mercury in modern industry
Mercury has a number of unique properties, which allows it to be valuable in almost any production. Here is a list of industries where this metal is used:
- Chemical industry. The largest amount of mercury is used in the production of chlorine components; it acts as a cathode in the production of sodium and chlorine. Mercury is also used as a catalyst in the formation of certain compounds.
- In nuclear energy, this metal is used to dissolve uranium. Mercury also helps with the thermochemical reaction of splitting water into oxygen and hydrogen.
- Metallurgical industry: here, with the participation of mercury, a number of important alloys are formed, necessary for engraving, lithography and electroplating. This area also appreciates the ability of mercury to absorb many metals, forming amalgams.
- Mercury compounds are often used in the production of precious metals.
- Electrical production: fluorescent, quartz and fluorescent lamps, current rectifiers using a liquid mercury cathode, batteries. Dry batteries, which use mercury in their production, power modern hearing aids.
- Heavy engineering industry: various vacuum installations, modern mercury diffusion pumps, heavily loaded hydrodynamic bearings, a large amount of mercury in liquid form is found in mercury-steam turbines.
- Even in astronomy this metal has found its use. In this field, the "horizon" device is often used, in which mercury is necessary to create the flawlessly mirror-like surface necessary for observing celestial objects.
- In the mining industry it is used to extract gold.
- Instrumentation: used for the manufacture of instrumentation equipment, barometers, thermometers; necessary for assembling washing machines, air conditioners and refrigerators.
- In petroleum refining, metal in the form of vapor is used to regulate the temperature required for refining.
- Mercury is widely used in medicine: it is involved in the creation of diuretics, antiparasitics and antiseptics. And in dentistry it helps in the manufacture of dentures and fillings from an amalgam of certain metals.
- Mercury compounds are also used in photography, leather processing, fabric dyeing, pyrotechnics, and porcelain production.
- In the military industry, liquid metal is used to make explosives, the so-called “mercury fulminate.” This substance is used in the detonators of shells and grenades.
- Shipbuilding. The paint used here contains mercury. It is used to treat the surfaces of ships under water. This paint, when interacting with sea chlorine, forms a film that kills harmful bacteria.
- In agriculture, mercury compounds are used as herbicides.
We learned about the purpose of using mercury in various industries.











