Yellow gold is a precious metal widely used in the jewelry industry. However, along with its remarkable properties - resistance to corrosion and oxidation, for example, the substance is extremely soft, and this makes it impossible to use it in jewelry in its pure form. Therefore, the necessary strength and wear resistance are achieved by adding impurities to the metal.
Physical and chemical properties

In chemistry, yellow gold (aurum) is a soft, ductile and very heavy metal with high thermal conductivity and low electrical resistance. One of its main features is the electronic structure of the atom, due to which the element absorbs photons in the short-wavelength part of the visible spectrum, and reflects long-wavelength quanta with lower energy. This is why gold appears yellow when illuminated with white light.
What makes this metal noble is the fact that under normal conditions it is chemically inert to most acids. An exception is aqua regia, which consists of concentrated aqueous solutions of nitric and hydrochloric acids in a ratio of 1:3. In addition, the reaction can occur with mercury and cyanide.
Product selection today
Gold is very popular now. During a crisis, people strive to invest money in eternal values, and, often, after thinking about it, they go to the bank for a bullion or investment coin. Jewelry can also serve as an investment. If desired, they can be sold to the same jewelry house where they were purchased, or taken to a pawnshop and wait for a professional assessment of its value.

Jewelry is mainly made for women, but there is also gold jewelry for men. A tie clip, watch bracelet, or cufflinks can be an excellent decoration and stylish addition to a businessman’s image. Products made of yellow gold in this case are perfect, because they can be pale enough so as not to stand out from the general image (in cases with 585 carat) and at the same time meet men’s requirements - simple, presentable, time-tested.

Often the mother of a child is faced with a choice - what earrings should she buy for her daughter to get her ears pierced? In this case, experimenting with alloys is dangerous, since the reaction of sensitive skin to additives can appear immediately and leave an unpleasant impression. You can buy stud earrings with a good clasp that covers the pin and protects the child’s neck from scratches at any jewelry store.

It is worth remembering that there are many fakes on the market. It could be an alloy that contains metals in the wrong proportion, an alloy that contains a very small percentage of pure gold, or it could be a fake that will begin to oxidize on the second day, reacting with water and your skin. In connection with these warnings, choose jewelry houses with a good reputation, and also ask for all documents confirming the sample of the product and its compliance with GOST.
Mining history
During the Neolithic era, the element was quite common in its native state, so people have been mining gold since time immemorial. This began in the Middle East; now gold mining is carried out in many countries. In Russia, the first reserves were discovered only in the 18th century, but now the state is one of the top three in global production volume.
The density of the noble metal is so high that it is comparable to tungsten, so its extraction is relatively simple. Obtaining is carried out using several methods:

- Washing with a stream of water, during which gold is concentrated in the heavy fraction of sand, and less dense minerals are washed away. The disadvantage is the loss of fine fractions, and sometimes quite large nuggets carried away by the flow.
- Amalgamation, based on the ability of mercury to form alloys with gold, which are then extracted from the total mass of the deposit. The method is used quite rarely, since it is applicable only with a high gold content in the ore.
- Cyanidation, which involves treating raw materials with cyanide compounds that dissolve gold. The precious metal is then deposited and sorbed in its pure form. The method is not applicable in ores containing sulfides and arsenides.
- Regeneration carried out by exposing the raw material to an alkaline solution and subsequent deposition of gold onto aluminum.
In addition, in the 20th century the dream of the alchemists was fulfilled. During experiments with mercury nuclei, part of it was transformed into gold. However, no one managed to get rich in this way, since such production is several times more expensive than mining from deposits.
Alloy features
Not only gold, but also many of its alloys are yellow in color, and this is the material that jewelers use in production. It's not just about the cost of the product when sold. The mechanical properties of gold in its pure form do not meet consumer requirements: products are easily deformed - the most minimal impact is enough, they are easily scratched and lose their configuration. Therefore, the development of alloys has a single goal - while preserving the color, to impart strength to gold products.
The composition may contain different proportions of precious metal, silver and copper; naturally, this will be reflected in the sample size. When choosing, you should remember that the higher the copper index in the alloy, the more saturated the color will be; the less copper there is, the paler the product.
What yellow gold is can be understood by considering the most popular alloys:
- fourteen parts of gold, seven parts of silver, three parts of red copper (585th standard);
- fourteen parts of gold, six – silver, four parts – red copper (585 standard);
- eighteen parts of gold, three and a half parts of silver, two and a half parts of red copper (750th standard).
So, the yellow color of gold objects does not mean 100% of the expensive metal in its pure form.
Cleanliness measures
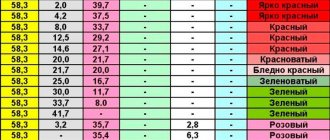
All alloys containing more than 30% noble metal must be assayed. That is, they must be marked with a mark that normatively fixes the proportion of the precious element. It can be determined using assay and spectral analysis, observing color changes under the influence of reagents, and reaction with aqua regia.
This is done by the Assay Office, which, along with the digital designation of the metal content, is required to apply a four-letter code, which can be used to determine the year of manufacture of the product, the institution that assessed it, and the manufacturer’s number.

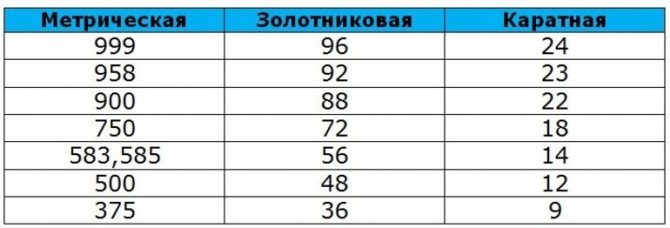
There are mainly four sampling systems:
- carat;
- metric;
- spool;
- lot.
The last two systems lost their relevance 100-200 years ago, others are still used successfully.
Carat system
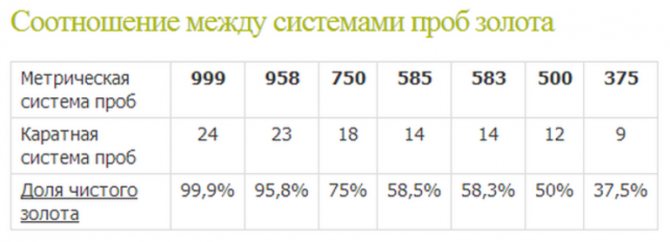
The carat is a common unit of mass measurement in the USA and Canada. It is equivalent to 200 mg. 24 carats is taken as 100% of the alloy being assayed; the hallmark of the precious metal depends on its proportion relative to the weight of the entire material. The discrete range of possible carat contents includes 9, 12, 14, 18, 21, 22, 23 and 24 carats. In the latter case, the weight of the noble metal exceeds 0.999 of the weight of the entire alloy, and the most popular and optimal value is 18 carats.
The karat standard is abbreviated as K, kt (from the word karat) or C, ct (from carat). Previously, submultiples such as ¼ or 1/16 carat were also used, but these have now fallen into disuse.
Metric standard
The metric system is now more widely used. Its value, along with the carat hallmark, must be stamped on jewelry made in Ireland. Most other countries use only the metric standard, but it is easily converted from carat. To do this, divide the number of carats by 24 and multiply by 1000.
The metric hallmark of yellow gold is an indicator of the pure substance content of the alloy . For example, if a piece of jewelry is stamped with the number 585, it means that a kilogram of the raw materials from which it was made contained 585 grams of the main precious metal.
The rest of the mass is occupied by impurities. Most often, 14k yellow gold contains silver and copper, but it may also contain additives of zinc, nickel, cobalt or palladium. Their purpose is to prevent damage and deformation of jewelry. In addition, they can be used to obtain colored gold. In addition to 585, gold can have a purity of 375, 500, 583, 750, 916, 958 and 999.
Yellow gold and its characteristics
Modern technologies make it possible to turn yellow gold into white gold. To do this, it undergoes special processing - immersion in rodage. The process itself is called rhodium plating. A thin layer of rhodium is applied to the surface of yellow gold. This improves the appearance of the metal, increases its strength and wear resistance.
Also, yellow metal differs from all others in its increased malleability. It will not be difficult to roll it into thin layers under high heat. Even in this form, it will not lose its properties and will retain its declared characteristics.
In the old days, the most popular was red gold. However, later they began to abandon it due to its excessive softness.
Gold of any color has a number of healing properties that make it more attractive and preferable compared to other metals:
- relieves inflammation;
- improves metabolic processes;
- relieves allergies;
- has a beneficial effect on the nervous system;
- improves memory and stimulates brain function;
- increases the body's endurance.
Treatment with gold does not require additional procedures. It is important to wear this metal constantly without taking it off.
You may be interested in: 750 gold standard - price per gram in a pawnshop
The many beneficial properties and qualities of this metal continue to make it the most popular in the jewelry market. However, many are interested in the question - which color of gold is better - red or yellow? What is the difference between them?
colored gold
By adding different impurities and varying their quantities, you can get many shades of the noble metal . It can be either well-known white and red gold, or exclusive green, purple, blue or black. In addition to color, mechanical properties, melting point, hardness and solderability change depending on the impurities. And the content of the main precious metal determines the product’s resistance to corrosion.
White and red
If you add whitening impurities to pure metal, you get white gold, which goes perfectly with diamonds and black pearls. Its similarity can be found in nature in the form of minerals called electrum. They are an alloy of gold with 16−69% silver.

In jewelry production, manganese and platinum can be used as impurities to impart white color, but an alloy of palladium and silver or nickel and zinc is more often used. The result of the first option will depend on the amount of silver. The more it is, the more matte the product will be. The second option is usually harder and more durable, so copper is added to it, increasing ductility. It does not affect the final color of the product, since its intense color is weakened by zinc.
The only drawback of an alloy that contains nickel is possible allergic reactions to this metal . When wearing the product for a long time, they appear in approximately 12.5% of cases and are usually limited to minor rashes on the skin. However, many manufacturers refuse to use nickel in jewelry and use palladium as a base. The pure white shine of the final product is achieved by coating it with rhodium using galvanization.
You can also make the gold pink or red. To do this, from 20 to 50% copper is added to the metal, which may also contain impurities of silver or zinc. The latter gives the product a yellow tint.
Red gold was known back in the Middle Ages; due to impurities, it acquired this color during the smelting process. It became especially popular in Russia at the beginning of the 19th century, but jewelry of this color is considered no less relevant now.
Green, purple, blue and black

Can make gold in less traditional shades. For example, add silver to it and get a greenish-yellow metal. This method was known back in 860 BC. A lighter shade of green is achieved by adding 23% copper and 2% cadmium instead of silver. If a dark green color is needed, the alloy includes 15% silver, 6% copper and 4% cadmium. Despite all the attractiveness of such an unusual shade of noble metal, it can be hazardous to health due to the toxicity of cadmium.
An alloy of gold with 15-21% aluminum makes the product purple. However, at the same time it becomes brittle and is easily destroyed by a sharp blow. Therefore, it is most often used as a gemstone. Malleable purple or, as it is also called, amethyst gold was obtained only at the end of the 20th century. It can be used as a structure, but the cost of the product will be quite high due to the very complex production process.
Gold, which is non-standard in both color and carat number, can be obtained by alloying it with 53.8% indium. The result will be a blue metal with a grayish tint weighing 11 carats. A more beautiful bluish tint and a weight of 14 carats can be obtained by using gallium as an impurity. However, such an alloy will be more brittle.

The best option for producing blue gold is considered to be air heat treatment of the surface of a metal alloy with iron and nickel. A thick 3-6 micron blue layer on a gold product can also be obtained by alloying ruthenium, rhodium and some other elements, followed by processing at a temperature of 1800 degrees Celsius.
With the help of specific surface treatment, black gold can also be obtained. For this, electrochemical methods using rhodium and ruthenium, chemical deposition with the participation of amorphous carbon, controlled oxidation of the alloy with chromium or cobalt, and the formation of sulfur and oxygen-containing films are used. The end result is brown and black jewelry.
Natural yellow
However, many prefer traditional yellow gold, in which the fineness exceeds the standard 585 g and is 750 g. It is called fire gold, has a rich hue and contains an increased amount of noble metal. The rest of the mass is represented by silver (170 g) and copper (80 g). The cost of such a material close to natural is quite high. You can sell it at a pawnshop for over 1,800 rubles per 1 g, and in a jewelry store you can buy it for no less than 4,500 rubles for the same mass.
Yellow gold, which has a purity of 585 g, has a more affordable price. Accordingly, there will be much more impurities in the form of silver and copper. This material is called lemon and can be purchased in the form of jewelry at a price of 2.5 to 3 thousand per gram.

Less typical for yellow gold is 958. In addition to the main component, it contains silver and copper. This material has a pleasant bright yellow color and is typically used to make wedding rings. But the finished product turns out to be quite soft, and the polish does not adhere well to it.
Another non-standard material for the production of wedding rings is 375-carat gold. Half of its composition is occupied by copper, the noble metal comes in second place, and there are also impurities of silver and palladium. Its main disadvantage is tarnishing in air due to the formation of silver sulfide.
999 purity gold is pure gold and can only be found in bullion. Its cost is very high, which, in particular, is due to the difficulties of obtaining such purity.
Composition of 585 standard and color difference of alloys
The purity of yellow gold is determined by the presence of a part of the noble metal in the material.
The most popular material for making jewelry is a material containing 58.5% pure gold, which corresponds to 14 weight units in the carat weighing system.
Depending on the composition of the additional material, its shade becomes white, red, yellow and pink. Yellow gold contains 18.75% silver and 22.75% copper.
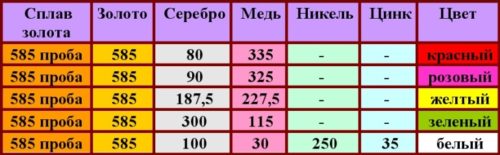
The chemically pure noble metal is very soft, and jewelry made from it is easily deformed. Additional physical properties obtained as a result of adding ligature components have a positive effect on the strength and stability of costume jewelry.
The presence of copper as an additional material gives the jewelry hardness and strength, but impairs its resistance to external factors, causing darkening on the surface.
Silver gives the precious component elasticity and reduces the melting point, which significantly speeds up the process of processing the material.
A small amount of platinum combined with gold can whiten the yellow color, and palladium can raise the melting point. Zinc increases the fluidity and hardness of the material, and nickel increases ductility. The yellow metal acquires a mirror-like surface and excellent anti-corrosion properties in an alloy with aluminum.
Application options
More than half of all gold reserves are in jewelry. The rest is distributed in the following areas:

- state and international financial organizations;
- investment savings;
- products from the electronics industry and dentistry.
As an element of the global financial system, metal is used due to the small volumes of reserves (and this despite the fact that throughout history it has practically not been lost, but only accumulated and melted down). The high mechanical properties and corrosion resistance of the metal make alloys containing it indispensable for the manufacture of electrical contacts in industry and implants in dentistry.
The advantages of red gold
Red gold also came to our age from antiquity. Previously, coins (chervonets) were mainly made from it. Today, modern jewelers quite often use red gold in their creations. Products made from it are relatively inexpensive, but nevertheless, they are not inferior in beauty to jewelry made from yellow gold.
The main advantages of red gold are:
- The ability not to lose shape and original appearance for many years.
- Copper, which is part of it, has a good healing effect on the human body.
- It has increased strength and durability, and is difficult to deform.
- Does not fade over time, does not lose its shine.
- The strength of red gold allows jewelers to create real works of art using it, and the flexibility of the material allows them to create the finest elements.
- The original color of red gold looks great on hands with gloves and rings without stones.
You may be interested in: Is it possible to reduce ring size at home?
Particularly strong alloys of red gold are obtained with the addition of silver. If there is a large amount of copper in it, undesirable oxidation processes are possible, which will lead to darkening of the metal and the formation of a brown film on its surface.