What is palladium
The chemical element is a plastic mineral having a silvery-white color. It is classified as a type of precious metal of the platinum group.
Brief history of appearance
Pd was first discovered in the 19th century. The chemical element was discovered by chemist William Wollaston (Great Britain). During the experiments, the scientist extracted it from platinum ore.
The metal received its name in honor of the asteroid Pallas discovered a year before. He, in turn, was named so thanks to the goddess from Ancient Greece Pallas Athena and her wooden image, the Palladium, which fell from the sky according to legend.
origin of name
Named after the asteroid Pallas, discovered by the German astronomer Olbers in 1802, that is, shortly before the discovery of palladium. In turn, the asteroid is named after Pallas Athena from ancient Greek mythology. Palladium, or Palladium, is the legendary wooden image of Pallas Athena that fell from the sky; according to the prophecy of Helen (son of Priam), Troy will remain indestructible as long as this talisman is kept within its walls. According to legend, only after the goddess’s favorites, Odysseus and Diomedes, stole Palladium during a night raid, did this stronghold fall.
What does palladium look like in nature?
Nuggets are not found in nature in their pure form. Metal particles are extracted along with other minerals. According to approximate data, there are approximately 30 elements that come into contact with palladium.
Externally, grains of the precious metal are very similar to platinum. In some deposits, these two elements are mined together (called palladium platinum) and then separated by chemical processing. Also, veins can intersect with gold, then a combination of two metals is observed (for example, palladium gold or porpecite from Brazil).
The process of formation in nature
The main source of appearance is space fragments of meteorites. A large content of precious metal crystals was found in iron and stone types of alien fragments.
Structure, chemical and physical properties
By its nature, the mineral compares favorably with other precious metals with its low density and chemical inertness. Thanks to the latter property, it does not interact with other elements and does not oxidize.
Reactions:
- Exceptions are silicon, boron, sulfur, chromium, with which palladium forms chemical compounds.
- Also, metal crystals dissolve in “regia vodka” (this is a mixture of two acids - sulfuric and nitric).
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
In appearance, the nuggets are similar to platinum and silver. The metal is very ductile, which is why it is actively used in jewelry. To improve strength and wear resistance, it is used in compounds with other metals.
The melting point is 1554 degrees Celsius.
Palladium history
Palladium discovery history
Mr. Forster, a well-known mineral merchant in London, did not express much surprise when, on one of the slushy autumn days of 1803, he received a letter from a person who wished to remain anonymous. On expensive paper, in beautiful handwriting, was a request: try to sell a small amount of the new metal palladium, neither in appearance nor properties inferior to precious platinum. Enclosed with the letter was a small and not very heavy ingot.
Forster agreed - the metal was really beautiful. Besides, nothing attracts people more than unusual and mysterious cases. And a merchant can benefit from them if he knows a lot about advertising. Soon, a report of a palladium ingot being sold in Forster's store became public, and passions flared up around the new metal.
Since the method of announcing the discovery of a new metal (through a merchant!) was clearly unusual, many scientists in England suspected a catch. The debate around palladium became increasingly heated both in the scientific community and among entrepreneurs.
At one time, Richard Chenevix stood out among English analytical chemists, most of whom were traditionally prim or phlegmatic. An Irishman by birth, a quick-tempered and quarrelsome man, he was especially eager to expose the “fraudulent trick” and, disregarding the high price, bought a palladium bar and began to analyze it.
Prejudice took its toll: very soon Chenevix became convinced that the metal called palladium was “not a new element, as was shamefully claimed,” but just an alloy of platinum and mercury. Chenevix immediately expressed his opinion - first in a report read before members of the Royal Society of London, and then in print. However, other chemists, with all their efforts, could not find either mercury or platinum in palladium.
The secretary of the Royal Society (founded back in 1660 and serving as the English Academy of Sciences) at that time was William Hyde Wollaston. A passionate opponent of routine and template in science, he from time to time intervened in a protracted dispute and skillfully aggravated it. Passions around palladium heated up and then waned, and when, finally, everyone began to get tired of the new element (or pseudo-element), an anonymous announcement appeared in the most famous scientific journal in England (Nicholson's Journal). The applicant, through the editor, offered a reward of £20 sterling to anyone who could prepare artificial palladium within a year. Interest in the new metal has surged again. But all attempts to artificially prepare palladium invariably ended in failure.
Only in 1804 did Wollaston report to the Royal Society that they had discovered palladium and another new noble metal, rhodium, in raw platinum. And in February 1805, in an open letter published in (Nicholson's Journab), Wollaston admitted that the scandalous hype around palladium was also his doing. It was he who put the new metal on sale, and then established a premium for its artificial preparation. And by that time he already had irrefutable evidence that palladium and rhodium were really new platinum-like metals.
While working to further improve the methods of refining and processing platinum, he came to the idea of the possibility of the existence of platinum-like metals. The commercial platinum Wollaston worked with was contaminated with gold and mercury. In an effort to obtain a purer metal, Wollaston got rid of these and other impurities. He dissolved raw platinum in aqua regia, then precipitated only platinum from the solution - with especially pure ammonia NH4Cl. It was then that he noticed that the solution remaining after the deposition of platinum was pink. This color could not be explained by known impurities (mercury, gold).
Wollaston reacted with zinc on the colored solution: a black precipitate formed. After drying it, Wollaston tried to dissolve it in aqua regia. Some of the powder dissolved, while some remained undissolved. About his further research, Wollaston wrote: “After diluting this solution with water, in order to avoid precipitation of small quantities of platinum remaining in the solution, I added potassium cyanide to it - a copious orange precipitate was formed, which when heated became gray... Then this precipitate fused into a droplet in specific gravity less than mercury... Part of this metal was dissolved in nitric acid and had all the properties of palladium put on sale.” Another platinoid, rhodium, was isolated from the other insoluble part.
Why did Wollaston call the first of the discovered satellites of platinum palladium, and the second - rhodium? Rhodium - from Greek - “pink”; Rhodium salts give the solution a pink color. The second name is not related to chemistry. It demonstrates Wollaston's interest in other sciences, in particular astronomy. Shortly before the discovery of palladium and rhodium (in 1802), the German astronomer Olbers discovered a new asteroid in the solar system and named it Pallas in honor of the ancient Greek goddess of wisdom Athena Pallas. And Wollaston named one of (his) elements in honor of this asteroid, more precisely, in honor of this astronomical discovery.
Wollaston had to extract palladium from raw platinum, incidentally mined during the washing of gold-bearing sands in distant Colombia. At that time, grains of native platinum were the only mineral known to people that contained palladium. Now many minerals are known that contain this element.
Like all platinum group metals, palladium is quite rare. Although what to compare with! It is estimated that there is 1-10-6% of it in the earth's crust, i.e. approximately twice as much as gold. The most famous placer deposits of platinum metals, and therefore palladium, are located in our country (Ural), Colombia, Alaska and Australia. Small traces of palladium are often found in gold sands.
But the main supplier of this metal was the deposits of nickel and copper sulfide ores. And, naturally, when processing such ores, precious palladium is extracted as a by-product. Extensive deposits of such ores are found in the Transvaal (Africa) and Canada. The deposits of copper-nickel ores in the Arctic (Norilsk, Talnakh) explored in recent decades have opened up opportunities for further increasing the production of platinum metals and primarily palladium. After all, its content in such ores is three times greater than platinum itself, not to mention its other satellites.
Methods for obtaining pure palladium from natural raw materials, based on the separation of chemical compounds of platinum metals, are very complex and time-consuming. Foreign companies involved in refining are not very willing to share their production secrets. Naturally, we do too. And it hardly makes sense to describe technology that was thirty years old. Therefore, let’s leave technology aside and let’s talk in more detail about minerals.
Of the six platinum metals, besides platinum itself, only palladium occurs in the native state. In appearance it is quite difficult to distinguish it from native platinum, but it is much lighter and softer than it. Chemical analysis shows that native palladium usually contains impurities: primarily platinum itself, and sometimes also iridium, silver and gold. But native palladium is extremely rare.
Minerals containing element No. 46 are its compounds with lead, tin (intermetallic compounds), arsenic, sulfur, bismuth, tellurium. About a third of these minerals have not yet been sufficiently studied and do not even have names. This is explained by the fact that minerals of all platinum metals form microinclusions in ores and are difficult to access for research. An excellent device, an X-ray microanalyzer, helped decipher the composition of some of these. It can be used to determine the chemical composition of samples weighing only 10~14 g!
One of the interesting minerals of element No. 46 is allo-palladium, the nature of which is still being studied. This silver-white mineral with a metallic sheen is very rare. Spectral analysis revealed that it contains mercury, platinum, ruthenium, and copper. But it has not yet been possible to definitively decipher the composition of this mineral. Palladium platinum was discovered in the ores of Norilsk. Its composition, identified using a microanalyzer, contains 40% palladium.
Back in 1925, the mineral potarite was found in the diamond deposits of British Guinea. Its composition PdHg was determined by conventional chemical analysis: 34.8% Pd and 65.2% Hg. However, the existence of other palladium compounds with mercury is also possible, for example Pd2Hg3.
In Brazil, in the state of Minas Gerais, a very rare and still insufficiently studied variety of native gold was found - palladium gold (or porpecite). It contains only 8-11% palladium. In appearance, this mineral is difficult to distinguish from pure gold.
These are some of the palladium minerals. By the way, palladium was also found in meteorites: 1.2-7.7 g/t of the substance in iron meteorites and up to 3.5 g/t in stone ones. And it was discovered on the Sun simultaneously with helium back in 1868.
Article on palladium history
How are palladium veins found?
Mineral inclusions are sought primarily in the locations of silver, copper and nickel ores. Occasionally there are small deposits with nuggets of pure metal.

Palladium satellites
In the bowels of the earth, palladium is found exclusively in the form of compounds with other minerals. Some of them have been little studied to this day and have no name. The most famous satellites of the precious metal are:
- braggite;
- palladite;
- potarit;
- stannopalladite.
It is also often extracted from gold and platinum veins.
Where does palladium occur in nature?
Under natural conditions of the earth's interior, the mineral is found in the form of compounds of different metals. Similar veins are found in Europe, the Russian Federation, and America.
Extraction methods
Work with palladium deposits is carried out in two forms:
- closed (mine);
- open (career).
In the first case, a system of underground tunnels - mines - is created for the extraction of precious metals. Small holes are created in the found ore layer, into which explosives are then placed. The soil loosened by the explosion is processed mechanically or manually to extract palladium particles. Once the initial refining is complete, the ore is transported to the surface and then transported to further processing.
In the second case, heavy earth-moving equipment and vehicles are used to transport the extracted ore. With its help, a soil quarry is developed, from which palladium is then extracted. It is then transported for processing to the appropriate enterprises.
Areas of application
The element widely uses:
- Chemical industry. Pd is a popular catalyst in oil refining and fat refining. Palladium chloride is also involved in the search for trace amounts of carbon monoxide in the air or gas mixtures. In electrochemistry, the same compound is an activating substance in the galvanic metallization of dielectrics. Palladium membranes are needed for hydrogen purification.
- Electrical engineering. The metal is important as a coating that is resistant to sulfides: the manufacture of high-precision voltagometers. Its physical characteristics have led to its use in the production of ceramic capacitors.
- Jewelry making. Palladium is added to products to create white gold. Even a small metal content in the alloy changes the shade of the item from yellow to silver-white. Occasionally, the mineral is used in the manufacture of commemorative coins.
- Medicine. Palladium is added to drugs intended to combat tumors and cancer therapy. Another area where metal is used is dentistry. Here, dentures are made on its basis. Alloys with the addition of palladium are used to create individual parts of pacemakers and medical instruments.
Chemical properties
Palladium is the most reactive of the platinum metals. Does not react with water, diluted acids, alkalis, or ammonia solution. Reacts with hot concentrated sulfuric and nitric acids, unlike other platinum metals. Can be brought into solution by anodic dissolution in hydrochloric acid:
At room temperature it reacts with aqua regia, with wet chlorine and bromine. When heated, it reacts with fluorine, sulfur, selenium, tellurium, arsenic and silicon. It is oxidized when fused with potassium hydrogen sulfate KHSO4, and also interacts with molten sodium peroxide.
When heated in air, it is stable up to ~300 °C and above 850 °C; in the range of 300...850 °C it fades due to the formation of a film of palladium oxide PdO, which decomposes at higher temperatures.
The richest deposits
Although large amounts of palladium are found in pieces of meteorites that fall to earth, the bulk of production comes from ore deposits. They provide about 98% of the world's metal reserves.
In the world
The Bushveld Complex (South Africa) is the world's largest palladium deposit. Here, prospectors find up to 40% of the world's precious metal reserves.

In much smaller quantities it is also extracted into:
- Lac des Iles (Canada);
- Stillwater (USA);
- Great Dike (Zimbabwe).
In Russia
The copper-nickel deposits that are part of OJSC MMC Norilsk Nickel are the largest metal suppliers in Russia:
- Oktyabrskoe;
- Talnakhskoe;
- Norilsk-1.
Their total profit is more than 40% of the global one.

Advantages and disadvantages of metal
First of all, the demand for palladium is determined by its physical characteristics:
- Compared to platinum, it has less weight, and therefore jewelry based on it, even large ones, are not heavy at all. Moreover, its strength is much higher than that of gold. This allows it to be used as a setting for large jewelry stones. Over time, such decorations do not darken and do not lose their attractiveness.
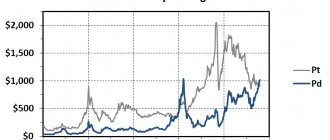
- Another undeniable advantage of palladium is its incredible external similarity to platinum. At the same time, its cost is much lower. According to rough estimates, the cost of 1 gram of metal is 2-3 times lower than that of gold or platinum.
As part of the alloy, palladium is resistant to wear, deformation, and scratches. However, pure metal has exactly the opposite properties, and therefore is used in rare cases, for example, for making exclusive jewelry. Palladium-based wedding rings are especially in demand. It is often used instead of nickel, providing a similar effect without causing an allergic reaction.
Where is star metal found?
Our current hero is a rarity. Data on palladium reserves vary: some analysts say that it is 2-3 times more than gold, others are sure that the mineral occurs in nature 20 times less often than gold.
In nature, the formation of palladium (solid solution) in native platinum is frequent. The silvery metal is found in the form of grains in platinum placers.
Minerals of palladium-containing ores:
- kotulskite;
- michenerite;
- merenskyite;
- atokit;
- Zvyagintsevit.
In Russia there are deposits of the “brother of platinum” in the Urals (Nizhny Tagil); the Konder ridge in the Khabarovsk Territory is ready to share silver metal; The Norilsk deposits of the Krasnoyarsk Territory are a complete basket of platinum group metals.
1 gram of palladium at the rate of the Central Bank of Russia costs about 5,200 rubles (as of April 2021). We recommend: LITHIUM - in space, on earth, under water
We are proud: Russia produces about 60% of stellar platinum.
Goldschmidt's classification classifies the metal as a siderophile.
Where to find the "platinum brother"
Sources of palladium in the world:
- SOUTH AFRICA. The famous Bushveld complex, there is an unimaginable amount of minerals and ores. Platinum group metals (almost 90% of the world's reserves), chromium, vanadium, iron, titanium... Palladium produces 20% of the world's production here.
- Australia, New South Wales. Mining is carried out from nickel ores.
- Zimbabwe. Here, in the Great Dyke, a huge ore-bearing area of chromite and platinum minerals, palladium veins are found.
- Brazil, Minas Gerais state. Placer deposits.
- Canada, Ontario. Millions of years ago a huge meteorite fell here, now people collect “gifts” - platinum group metals, nickel. Mining is carried out by open-pit mining.
- Colombia, Chocó province. Placer platinum, gold, palladium.
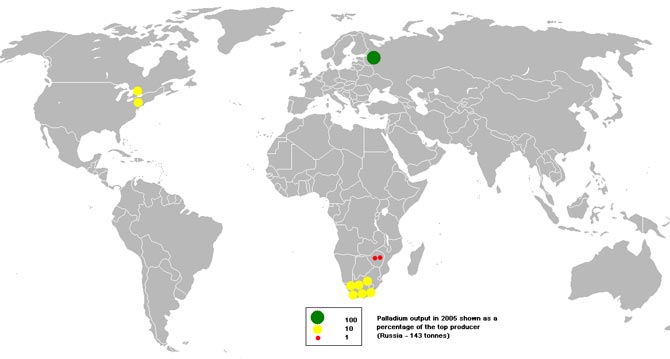
Main palladium mining areas
Types of alloys and samples
Since palladium in its pure form is too soft, alloys based on it are used to make jewelry.

In Russia, two samples are legally approved - 500 and 850. The alloys used are nickel, silver and copper. 950 standard is also popular abroad. In this case, 95% palladium accounts for 5% copper or ruthenium additives. Occasionally they are replaced with nickel to provide greater strength to the alloy.
Sample correspondence table
Palladium alloys approved in Russia are prescribed in GOST. The composition and amount of ligature in each of them can be tracked using the table presented here.
| Try | Mass fraction of component, % | |||
| Palladium | Silver | Nickel | Copper | |
| 500 | 50,0 | 44,5 | 5,5 | — |
| 850 | 85,0 85,0 | 12,5 — | 2,5 — | — 15,0 |
Interesting Facts
- Palladium is a precious metal and is traded on exchange and over-the-counter markets. In some countries, including Russia, legislation allows individuals and legal entities to open “metal accounts” in palladium in banks.
- In the Russian Federation, for the illegal acquisition, storage, transportation, shipment and sale of palladium (as well as other precious metals gold, silver, platinum, iridium, rhodium, ruthenium and osmium) in a large amount (that is, worth more than 2.5 million rubles. ) with the exception of jewelry and household products and scrap of such products, criminal liability is provided in the form of imprisonment for a term of up to 5 years.
How to spot a fake
It is almost impossible to distinguish palladium from other metals by eye.
If there is any doubt about the authenticity of the metal, it is recommended to show it to an independent jeweler appraiser. You can be sure that you are holding real jewelry in your hands if over time they have not lost their attractiveness and shine. If the jewelry begins to darken, this is definitely a fake.
Tips for choosing palladium jewelry
Make sure that the jewelry is marked with an indication of the purity (500 or 850).
The silvery-white color of the metal goes better with diamonds, sapphires, amethysts, labradorite and aquamarines.

When choosing wedding rings, pay attention to the shape of the inner surface. For comfortable wearing, it should be slightly curved.
Physical properties
Palladium is a transition metal. Under normal conditions, it forms silvery-white crystals of cubic crystal structure, space group Fm
3
m
, cell parameters
a
= 0.38902 nm,
Z
= 4, structural type of copper.
Palladium is plastic; microadditives of nickel, cobalt, rhodium or ruthenium improve the mechanical properties of Pd and increase hardness.
Insoluble in water. Density - 12.02 (20 °C, g/cm³); under special conditions it forms colloidal palladium and palladium black. Melting point - 1554 °C (in some sources 1552 °C); boiling point about 2940 °C. The heat of fusion is 16.7 kJ/mol, the heat of evaporation is 353 kJ/mol. Specific heat at 20 °C - 25.8 J/(mol K); electrical resistivity at 25 °C - 9.96 μΩ/cm; thermal conductivity - 75.3 W/(m K). Vickers hardness 37...39. Brinell hardness 52 kgf/mm 2.
Temperature coefficient of linear expansion 1.17·10 −5 K −1 (in the range 0...100 °C).
The surface tension coefficient of liquid palladium at the melting point is 0.015 N/cm.
Palladium is paramagnetic; its magnetic susceptibility is +5.231·10 −6 (at +20 °C).
Actively absorbs hydrogen, forming solid solutions (up to 900 volumes of H2 per volume of Pd), while the lattice constant increases. Hydrogen is removed from palladium by heating to 100 °C in a vacuum.
Isotopes
Natural palladium consists of six stable isotopes: 102 Pd (1.00%), 104 Pd (11.14%), 105 Pd (22.33%), 106 Pd (27.33%), 108 Pd (26.46 %) and 110 Pd (11.72%).
The longest-lived artificial radioactive isotope 107 Pd ( T
1/2 7 10 6 years). Some isotopes of palladium are actively produced as fission fragments of uranium and plutonium; Thus, the irradiated fuel of modern reactors with a burnup degree of 3% contains 0.15% palladium.
What radio components contain
In radio engineering, palladium is often found in the following parts:
- connectors;
- capacitors;
- resistors.
First of all, this concerns devices that are important in the military and space industries. In civil engineering, palladium is used only in aviation.
How to distinguish palladium from platinum in radio components
At home, distinguishing two precious metals is difficult, but possible. The easiest way is to drop a small sample into a container of nitric acid. If the metal dissolves, you have palladium.
Another method involves using a touchstone, potassium iodide and aqua regia. A metal sample is passed along the edge of the stone until a scratch is formed. Then a mixture of potassium iodide and aqua regia is poured into it. If the scratch is painted red with a brown tint, we can say that the presented sample is palladium.
Ways to isolate metal
Options:
- Electrolytic reaction. Refining involves the use of sulfuric acid, which will separate the palladium compounds, leaving the brass and copper elements intact. Aqua regia will help extract pure metal after the reaction is complete.
- Ammonia solution and hydrochloric acid also help to isolate palladium. The color of the precious metal plays an important role in the refining process. For example, brown confirms the presence of palladium in the alloy.
Also watch the video below about what else you can get palladium from:
What kind of light platinum is it?
Place of palladium in group 10 of the periodic table of elements of the periodic table.
Physical properties of metal:
- Density 12 g/cm3.
- Of all the platinoids, this is the most fusible metal - 1552 degrees Celsius.
- The metal color is stable, silver, white.
- Palladium is a very soft metal, but adding ruthenium or nickel to the alloy significantly increases its hardness.
- Palladium can accumulate hydrogen in large quantities, especially in a finely divided state.
The lattice structure is face-centered.
The precious metal has a copper structure (Bravai structure).
The chemical properties of palladium are somewhat different from those of the platinoids. Of the entire family, the metal is the most chemically active. It reacts with hot concentrated acids (H2SO4, HNO3 - sulfuric, nitric).

But palladium does not react to dilute alkalis and acids.
According to the characteristics, palladium is close to its neighbors on the periodic table. The luster and color are close to silver; its chemical properties are similar to platinum.
The easiest way to distinguish palladium from platinum is by weight. The densities of these metals differ by almost two times. Outwardly, both metals are similar, like twins.
| Properties of the atom | |
| Name, symbol, number | Palladium (Pd), 46 |
| Atomic mass (molar mass) | 106.42(1)[1] a. e.m. (g/mol) |
| Electronic configuration | [Kr] 4d10 |
| Atomic radius | 137 pm |
| Chemical properties | |
| Covalent radius | 128 pm |
| Ion radius | (+4e) 65 (+2e) 80 pm |
| Electronegativity | 2.20 (Pauling scale) |
| Electrode potential | +0,987 |
| Oxidation states | 0, +1, +2 (most common), +3, +4 (common), +5, +6 (very rare) |
| Ionization energy (first electron) | 803.5(8.33) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 12.02 g/cm³ |
| Melting temperature | 1554 °C |
| Boiling temperature | 2940 K |
| Ud. heat of fusion | 17.24 kJ/mol |
| Ud. heat of vaporization | 372.4 kJ/mol |
| Molar heat capacity | 25.8[2] J/(K mol) |
| Molar volume | 8.9 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 3.890 Å |
| Debye temperature | 274 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 71.8 W/(mK) |
| CAS number | 7440-05-3 |
We recommend: SILICON - everyone needs it
Reviews
Katerina: “It just so happens that I love jewelry, especially rings. Knowing this passion of mine, my family constantly gives me something new. I also have several rings, both palladium and platinum. And I like the first ones much more. Firstly, they are lighter and do not feel much when worn. Secondly, I like their color better. Well, if you buy it yourself, palladium rings are much cheaper than platinum.”
Svetlana: “Palladium jewelry is good for everyone, except for one thing - it’s almost impossible to find them. I had to scour more than one store and online resources to find what I needed. In theory, it was possible to buy the same white gold based on palladium, but in fact very few of them are made. Basically, everything is a regular gold alloy with rhodium plating. I wish there was a better choice."