Jewelers call platinum the queen of precious metals. But it was not always so. Until the 18th century, it was not mined on an industrial scale, and was even called “bad silver.” Let's figure out what platinum is and what its value is. We will learn many interesting facts about what it looks like in its original form, how it is mined and used.
If you are the happy owner of platinum jewelry or just want to become one, let’s find out how to “not fall for” a fake.
What is platinum
This is a silver-colored precious metal. Outwardly it resembles silver, but has completely different physical and chemical properties.
Platinum deposits are rarely found in nature. Their development is extremely labor-intensive. For these reasons, the price of the metal on the market is even higher than gold.
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
Platinum (as denoted in chemistry, Pt, platinum) is one of the elements of the periodic table. This is a dense, hard, but very plastic material.
Brief history of appearance
The ancient Egyptians, Incas and Chibcha tribes used the metal as jewelry. Platinum came to the European continent along with Spanish sailors from South America. The precious metal was not appreciated at that time. Even the word platina translated from Spanish sounds like “dirty silver.” Since it has a high density and is refractory, it was considered unsuitable for consumption. Often they even threw it away.
Fraudsters were the first to use Pt in jewelry. It was added to gold alloys, increasing the weight of the item without reducing the cost. Production reached such a scale that the import of platinum into Europe was banned.
Only in the middle of the 18th century the metal was isolated as a separate chemical element. Properties were studied - operational, physical. At the turn of the 19th century, scientists discovered that platinum is not just a noble metal, but also serves as the “mother” for a whole family of platinum group metals:
- palladium;
- rhodium;
- osmia;
- iridium.
Platinum as a type of metal
Platinum belongs to the group of elements of the same name and the 10th group of the periodic table of elements. This metal has 6 naturally occurring isotopes. Platinum is one of the rarest elements that make up the earth's crust (density is about 5 μg/kg). Platinum is found in some varieties of copper and nickel ores or directly in native platinum ore. 80% of the world's production of this metal comes from South Africa. Due to the low content of platinum ore within the earth's crust, a relatively small amount of this metal is produced annually. It is in great demand in some areas of activity.
Platinum is found in such ore.
Platinum is a chemically low-active metal. It has exceptional corrosion resistance even at high temperatures, which is why it is considered noble. Platinum is very often found in its native form, which is associated with low chemical activity. It occurs naturally in various sandy rivers of South America. In the times before the discoveries of Christopher Columbus, local people used platinum to make artifacts. This information first appeared in European publications from the early 16th century. In 1748, an official report on an unknown metal native to Colombia was published, after which it began to be studied in detail by scientists.
Platinum is often used in catalytic converters, laboratory equipment, contacts and electrodes, thermometers, dental instruments and jewelry. As a heavy metal, platinum can cause health problems in humans due to its salts. But due to its resistance to corrosion, it is not as toxic as many other metals. Compounds containing platinum (cisplatin, oxaliplatin and carboplatin) are used in chemotherapy to treat certain types of cancer.
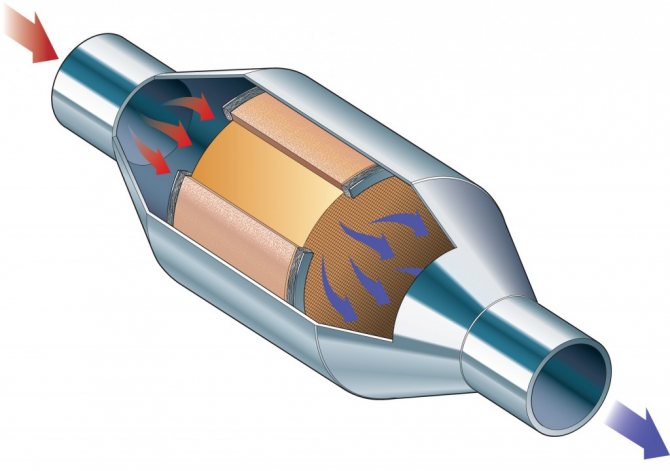
Platinum is used in automobile catalysts.
Of the 245 tons of platinum sold in 2010, 113 tons were used to produce emission control devices in automobile exhaust systems (46%). 76 tons were needed to make jewelry (31%). The rest was used mainly to make electrodes, cancer drugs, oxygen sensors, spark plugs and gas turbine engines.
What does platinum look like in nature?
It is not found in its pure form in natural conditions. Forms isomorphic mixtures with iron, copper, silver, nickel, and platinum group metals. Platinum-containing ore has small grains and inclusions of precious metal.

Native metal is considered to be mined nuggets containing from 75 to 92% Pt. They are rarely found. Ferrous platinum (polyxene), which contains 20-50% iron, is mainly mined.
The process of formation in nature
Platinum ores are in a dispersed state. They are of igneous origin, released through the crystallization of basic and ultrabasic magmas. At a temperature of 1300-1500 degrees, sulfides, platinum, chloride, osmium, and iridium are separated from the silicate melt.
The surface of bedrock deposits is destroyed over time. The resulting placers are convenient for industrial development.
Structure, chemical and physical properties
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
The structural structure of the crystal lattice is a cube with third-order symmetry elements. The metal has refractoriness, low thermal conductivity, and high density (21.45 g per dm2). Melting point - 1769 degrees, boiling point - 3800.
Hard material is difficult to process. It is so durable that jewelry can be made from pure platinum without adding impurities.
Other physical and chemical properties:
- plasticity when heated (you can make the thinnest foil or wire);
- resistance to corrosion, oxidation;
- lack of interaction with acids and alkalis;
- low resistivity (serves as a good conductor);
- catalyst for many chemical reactions.
Also watch the video for other properties of platinum:
Physical properties
Grayish-white ductile metal, melting and boiling points - 2041.4 K (1768.3 °C) and 4098 K (3825 °C), respectively, electrical resistivity - 0.098 μOhm m (at 0 °C). Platinum is one of the heaviest (density 21.09–21.45 g/cm³; atomic density 6.62⋅1022 at/cm³) metals. Brinell hardness is 50 kgf/mm2 (Mohs 3.5).
The crystal lattice is face-centered cubic, and
= 0.392 nm,
Z
= 4, space group
Fm
3
m
.
Platinum is resistant to vacuum and can be used in space technology.
How are platinum veins found?
The main place of production is copper and nickel deposits (primary and alluvial). From them, platinum is mined along with other metals. Platinum nuggets are found in ultramafic igneous rocks. Natural mineral ores with high element content are rare.
Satellites of platinum
In deposits, platinum accompanies platinum group metals.
In addition, in various rocks, platinum is found with the following accompanying minerals:
- Basic and ultrabasic magmas—serpentine, chromite, magnetite, chrysotile-asbestos, olivine, orthorhombic pyroxenes.
- Placers - chromite, corundum, magnetite, gold, diamonds.
- Diabase - chalcopyrite.
Where is platinum found in nature?
This is the rarest of the elements of the Earth's crust.
Found as nuggets, alloys with nickel, copper, and platinum group metals.
Deposits where platinum is found are associated with mafic and ultramafic igneous rocks.
History of platinum
Platinum group metal alloys (PGMs), which include platinum, were used to decorate the Casket from Thebes, an Egyptian tomb that dates back approximately 700BC. This is the earliest known use of platinum, although pre-Columbian South Americans also made jewelry from gold and platinum alloys.
Platinum wheels.
The Spanish conquistadors were the first Europeans to encounter the metal, although they were uncomfortable with the pursuit of silver due to its similar appearance. They named the metal Platina—a version of Plata, the Spanish word for silver—or Platina del Pinto because of its discovery in the sands along the banks of the Pinto River in modern-day Colombia.
Despite being studied by a number of English, French and Spanish chemists in the mid-18th century, François Chabanot was the first to produce a pure sample of platinum metal in 1783. In 1801, Englishman William Wollaston discovered a method for efficiently extracting the metal from ore, which is very similar to process used today.
The silvery appearance of the platinum metal quickly made it a prized commodity among royalty and the wealthy who sought jewelry from the latest precious metal.
Growing demand led to the discovery of large deposits in the Ural Mountains in 1824 and in Canada in 1888, but the discovery that would radically change the future of platinum did not come until 1924, when a farmer in South Africa tripped over a platinum nugget in a riverbed. This eventually led to geologist Hans Merensky's discovery of the Bushveld magnesium deposit, the largest platinum deposit on Earth.
Although some industrial applications for platinum (such as spark plug coatings) were in use by the mid-20th century, most modern electronic, medical, and automotive applications have only been developed since 1974, when U.S. air quality regulations initiated the autocatalyst era.
Since then, platinum has also become an investment vehicle and is traded on the New York Mercantile Exchange and the London Platinum and Palladium Market.
Top countries by production
The leading countries in the world market are:
- Republic of South Africa.
- Russia.
- Zimbabwe.
- USA.
- Canada.
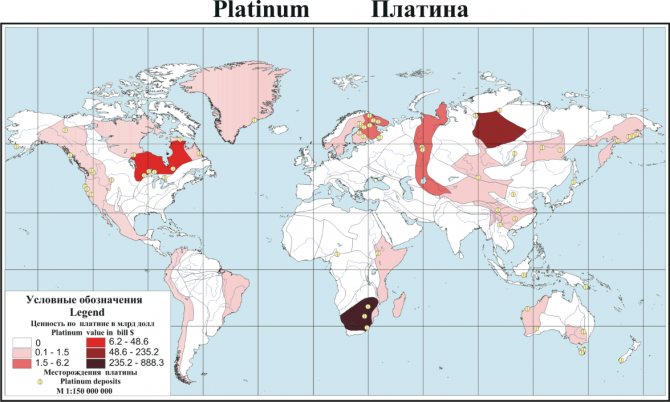
World platinum reserves
80% of platinum group metal deposits available for development are located in South Africa (Bushveld complex in the north of the country).
The second largest reserves are the GreatDyke field (Zimbabwe).
3rd and 4th place in the Russian Federation and North America (USA, Canada). Canadian platinum ores are concentrated in the provinces of Ontario and Manitoba. In the United States, the bulk of production occurs in two large mines in Montana.
Colombian placer deposits are saturated with platinum group metals. They are concentrated in the western Cordillera, in the river valleys of Atrato and San Juan.
Production
Platinum is one of the most expensive metals, its price is 3-4 times higher than gold, and about 100 times higher than silver
Platinum production is about 36 tons per year. The largest amounts of platinum are mined in Russia, South Africa, Kaiada, the USA and Colombia.
In Russia, platinum was first found in the Urals in the Verkh-Isetsky district in 1819. When washing gold-bearing rocks, white shiny grains were noticed in the gold, which did not dissolve even in strong acids. Bergprober of the laboratory of the St. Petersburg Mining Corps V.V. Lyubarsky examined these grains in 1823 and found that “the mysterious Siberian metal belongs to a special kind of raw platinum containing a significant amount of iridium and osmium.” In the same year, the highest order was issued to all mining chiefs to look for platinum, separate it from gold and present it to St. Petersburg. In 1824-1825, pure platinum placers were discovered in the Gorno-Blagodatsky and Nizhne-Tagil districts. And in the following years, platinum was found in several more places in the Urals. The Ural deposits were exceptionally rich and immediately brought Russia to first place in the world in the production of heavy white metal. In 1828, Russia mined an amount of platinum that was unheard of at that time - 1550 kg per year, about one and a half times more than was mined in South America for all the years from 1741 to 1825.
Extraction methods
The production process consists of three stages:
- Ore mining.
- Enrichment.
- Obtaining precious metal of high purity.
Extracting platinum from the earth's interior is a labor-intensive and expensive task. To extract 1 ounce (31.1 grams) of precious metal, more than 10 tons of ore are processed.
There are two ways to get it:
- open;
- underground.
The first option is suitable for placer deposits formed as a result of the destruction of primary rock. It involves the use of quarry equipment, dredges, and hydromechanical means.

Primary deposits and buried placers are mined underground. Shafts are dug, holes are manually drilled into them, and explosives are placed. Miners lift pieces of rock that break off to the surface for further processing. Today, this version of metal mining is significantly mechanized, but it cannot be done without manual labor.

Platinum in the chemical and oil refining industries
Platinum group metals are used in the chemical industry as catalysts to increase the efficiency of reactions.
Platinum is widely used as a catalyst in the production of nitric acid, which is the starting material for the production of nitrogen fertilizers and other substances.
Also, platinum catalysts are used in the production of various silicones. The addition of platinum to a silicone mixture catalyzes the “cross-linking” of silicone structures, making it possible to obtain a material with desired properties. Silicone is a very durable material with excellent resistance to chemical corrosion, heat and temperature changes. Silicones are also very flexible, waterproof and electrically insulating materials. The range of their applications is extremely wide - from aircraft engine parts to medical and cosmetic materials. It is clear that silicones will be used more and more in the future and, accordingly, the demand for platinum in this industry will increase.
Platinum catalysts are used in oil refineries to produce gasoline and petrochemical feedstocks, which are the basis for the production of plastics, synthetic rubber and polyester fibers. Oil entering oil refineries is a mixture of hydrocarbons, which belong to the heavy and light fractions. The ratio of fractions depends on the region of production, but in general there are more heavy fractions, while the light fraction is used to obtain gasoline and high-quality raw materials for further processing. Therefore, one of the main tasks of plants is to convert heavy fractions into light ones. This is achieved through a complex multi-stage process of oil distillation.
Platinum is involved in distillation steps such as reforming and isomerization, which produce high-octane components for gasoline. For reforming and isomerization, catalysts in the form of platinum-coated alumina beads or granules are used. In this case, the weight of pure platinum is no more than 0.6% of the weight of the catalyst. In most modern factories, platinum is used together with tin or rhenium to increase productivity. Platinum is the key to oil refining; without it, the process would be ineffective.

In the beginning, oil refining was one of the main areas of industrial consumption of platinum. But improvements in technology and the catalysts themselves have led to less and less platinum being consumed, despite the growing number of oil refining capacities. The volume of platinum consumption for this industry does not experience sharp changes.
Areas of application
The industries that use platinum are varied.
List of applications:
- oil and gas industry (production of high-octane gasoline and technical hydrogen from oil fractions);
- automotive industry (production of exhaust gas afterburning catalysts);
- electrical engineering (elements of high-temperature furnaces, mirrors for lasers, magnets);
- ammonia synthesis;
- chemical, glass industry (equipment with high resistance to chemical and temperature influences, electrodes, reaction catalysts);
- medical instruments;
- making jewelry.
Applications of platinum
For a metal whose annual global production is only 192 tons, platinum is found critical to the production of many everyday items.
The largest use, accounting for about 40% of demand, is in the jewelry industry, where it is primarily used in the alloy that produces white gold. It is estimated that more than 40% of engagement rings sold in the United States contain some platinum. The US, China, Japan and India are the largest markets for platinum jewelry.
Platinum's corrosion resistance and high temperature stability make it ideal as a catalyst in chemical reactions. Catalysts speed up chemical reactions without undergoing any chemical change in the process.
Platinum's main application in this sector, accounting for about 37% of total metal demand, is in catalytic converters for automobiles. Catalytic converters reduce harmful chemicals from exhaust emissions by initiating reactions that convert more than 90% of hydrocarbons (carbon monoxide and nitrogen oxides) into other, less harmful compounds.
Platinum is also used to catalyze nitric acid and gasoline; increasing octane levels in the fuel.
In the electronics industry, platinum crucibles are used to make semiconductor crystals for lasers, while alloys are used to make magnetic disks for computer hard drives and switch contacts in automotive controls.
Demand is growing in the medical industry as platinum can be used for both its conductive properties in pacemaker leads and ear and retinal tissue implants, as well as its anti-cancer properties in drugs (such as carboplatin and cisplatin).
Below is a list of some other platinum apps:
- With rhodium used to make high temperature thermocouples
- To make optically clear, flat glass for TVs, LCDs and monitors
- Making glass threads for fiber optics
- In alloys used to form the tips of automotive and aeronautical spark plugs
- As a replacement for gold in electronic connections
- In coatings for ceramic capacitors in electronic devices
- In high temperature alloys for jet fuel injectors and rocket nose cones
- In dental implants
- To make high quality flutes
- Smoke and carbon monoxide detectors
- For the production of silicones
- In razor coatings
More on the topic:
- Palladium: properties and applications Properties and applications of Palladium Get information about the properties, characteristics and applications of the palladium metal Palladium -…
- Application of galvanized steel Do you know what is the application of galvanized steel? In the past, most homes used galvanized steel...
- Tungsten (Tungsten): Properties, Production, Applications and Alloys Tungsten is a dull, silvery metal with the highest melting point of any pure metal. Also…
- Beryllium Metal: Applications Applications of Beryllium Metal Applications of Beryllium Metal Beryllium applications can be divided into five areas: Consumer Electronics…
Powered By
The richest platinum deposits
Total world reserves in known deposits are about 66 thousand tons. Most of them are located in South Africa (63 thousand tons). Russian deposits are rich in 1.1 thousand tons, American - 0.9 thousand tons, Canadian - 0.3 thousand tons, other countries - 0.7 thousand tons.
In the world
The largest deposits of platinum-containing ores are located in South Africa. These are ultramafic rocks from the Paleozoic era at Bushwell.

Other major world deposits:
- Sudbury (Canada);
- Nevada, California, Wyoming, Alaska (USA);
- Quibdo, Andagoda, Opogodo, Tamana, Condoto-Iro (Colombia);
- Norway, New Zealand.
In Russia
For the first time on the territory of the Russian Federation, deposits were discovered in the 20s of the 19th century in the Ural Verkh-Isetsky district.
Main platinum deposits:
- Oktyabrskoe;
- Talnakhskoe;
- Nizhny Tagil;
- sulfide-copper-nickel in Norilsk, Krasnodar region, Fedorova tundra, Zarechenskoye in the Murmansk region;
- alluvial deposits in the Khabarovsk Territory (Konder), in Kamchatka (Levtyrinyvayam), on the Lobva River, Vyysko-Isovskoye.
Platinum is native.

Until the beginning of the 18th century, platinum did not attract any attention: it was considered a white metal accompanying gold and not of particular value. But only in the Old World. In South America it has been highly valued since ancient times. In what is now Ecuador, platinum has been processed as a precious metal since at least the 1st century BC.
In 1520, King Charles V of Habsburg of Spain learned that the conquistadors had discovered significant accumulations of white metal in the gold deposits of the Pinto River (Colombia), which was highly valued by the local Indians. A little earlier, the Aztec emperor Montenzuma II sent him polished platinum mirrors as a gift.
But obviously these gifts from the overseas ruler did not make much of an impression on the Spanish monarch, since two hundred years later platinum in Europe was valued lower than silver. Hence the origin of the name, which literally means “little silver”: diminutive and disparaging from Spanish. "plata" - silver. At the beginning of the 18th century, counterfeiters learned to mix it with gold and in 1735, by decree of the Spanish King Philip V, platinum was prohibited from being imported into Spain, and about four tons of the precious metal were drowned in the sea.
The first samples of native platinum were brought from South America to Europe in 1746 by the Spanish astronomer Antonio de Ulloa (1716 - 1795). In 1752, they were studied by the Swedish chemist H. Scheffer, who was the first to describe platinum as an independent metal. Since native platinum is extremely rare in its pure form, he was dealing with a mixture of chemical elements, some of which were still unknown at that time and the description was very inaccurate.
Almost pure malleable platinum was first obtained in 1786 by the French chemist Pierre-Francois Chabaneau, who managed to remove most of the natural impurities from the mineral. And only then was it possible to fully appreciate the remarkable properties of this metal.
The chemical symbol is Pt. Syngony: cubic. The crystal structure is similar to that of copper and is a face-centered cubic lattice with a very close packing of atoms. In nature, platinum is very rarely found in its pure form. It usually forms an isomorphic mixture with other metals, both from the platinum and other groups.

Platinum iridide; nugget about 1 gram; North Svetlinskaya placer, Southern Urals. Photo: Yu. Khomyakov.
In fact, the term “native platinum” is a collective name for a number of mineral species.
Samples containing more than 80% Pt are usually considered native platinum. The most common ferrous platinum (fe content from 20 to 50%) is the so-called “polyxene” (Greek “polyxenos” - many aliens). Depending on the composition, many more varieties are distinguished: palladium platinum (Pd up to 40%), ~ rhodium (about 5% Rh), ~ iridium (Ir 10 - 37%), ~ nickel (about 3% Ni), norilskite or ferronickelplatinum ( up to 25% Fe and up to 25% Ni), cuproplatinum (up to 14% Cu), etc.
Typically found as interspersed grains or nuggets; well-formed crystals are extremely rare.

Native platinum; twin crystals about 2 cm; Konder ridge, Khabarovsk region. © Wendell Wilson
Color: from silver-white to steel gray. Gloss: metallic. There is no cleavage. Fracture: uneven, hooked. Hardness: 4 - 4.5; for iridescent platinum - up to 7. Specific gravity: 14 - 19; for pure platinum - 21.45 g/cm3. Feature: silver-gray with a metallic sheen.
Very malleable and malleable - you can forge sheets up to 0.0025 mm thick and draw wire with a diameter of up to 0.015 mm. Melting point: 1774°C. Dissolves only in heated aqua regia. Good electrical conductor. Ferrous varieties may have magnetic properties.
Forms in mafic magmas at temperatures around 1500°C when sulfides separate from the silicate melt. It occurs as inclusions in ultrabasic igneous rocks (dunites, peridotites, pyroxenites), as well as in copper-nickel sulfide accumulations of basic rocks (gabbro, diabase). But the most important industrial significance are placers that are formed as a result of the destruction of the surface layers of bedrock deposits. Associated minerals include: chromite, chrome diopside, olivine, serpentine, magnetite, sperillite; in placers it is also associated with corundum, diamond, and native gold.
In Russia, industrial mining of platinum began in 1825 - in gold placers near Nizhny Tagil. In the first 20 years, about 40 tons were mined here. In 1892, a primary deposit was discovered in the same places. The largest Ural platinum nugget weighing 11.5 kg and measuring 18 X 10 cm was found in the Syrkov Log mine. Of those that have survived to this day, the largest is the “Ural Giant” (7860.5 g), which is stored in the Diamond Fund of the Russian Federation. Well, the absolute world champion is probably a nugget weighing about 12 kg, found on the Konder ridge in the Khabarovsk Territory.
In 1790, French jeweler Marc-Etienne Janet used platinum for the first time, making a sugar bowl for King Louis XVI. Nowadays, this product is kept in the New York Metropolitan Museum of Art.
In Russia, platinum has long been known in the gold-bearing placers of the Urals, where it was nicknamed “frog gold” and was sometimes used instead of shot, and more often it was simply thrown into a dump. Methods for its processing were developed by metallurgical engineers Pyotr Grigorievich Sobolevsky and Vasily Vasilyevich Lyubarsky in 1827.
At first, chains and hoops for barrels were made from platinum. The first such products were preserved in the Mining Museum on Vasilyevsky Island in St. Petersburg (National Mineral Resources University "Mining"). From 1828 to 1845, on the initiative of the Minister of Finance Yegor Frantsevich Kankrin, platinum coins were minted in Russia.
The extraordinary lightness and subtlety of jewelry from the beginning of the last century would have been impossible if jewelers had not started using platinum. The relatively low hardness of silver led to an increase in the weight of the frame. For durability, as well as to avoid dark silver stains on skin and clothing, it was duplicated with gold. As a result, the products became heavy, uncomfortable and, moreover, faded over time.
The strength and hardness of platinum allowed jewelers to transform it into stunning pieces with lace or geometric patterns. In addition, the amount of metal in the frame could be reduced to a minimum, and there was no longer any fear that the jewelry would fade over time. Round platinum plates, processed using special saws and other tools, became an excellent basis for brooches and pendants with diamond flowers and garlands.

The high quality of platinum as a jewelry material was appreciated by the craftsmen of the Carl Faberge company. In the famous “Winter Egg”, a basket with flowers is made of platinum, encrusted with 1,378 diamonds. In 1912, after the Titanic disaster, Louis Cartier's platinum jewelry with black enamel and rock crystal came into fashion. Until the outbreak of World War II, platinum was a very popular jewelry material. Almost half of the total production was used for the production of jewelry.
In 1943, the Order of Victory was established in the Soviet Union. It is a star made of 47 grams of pure platinum. The ruby rays of the order are surrounded by diamonds weighing a total of 16 carats. The distance between the tops of opposite beams is 72 mm. It was practically impossible to find natural rubies of identical color, so artificial corundum had to be used (25 carats per order). The awards were made at the Moscow Jewelry and Watch Factory under the direction of master I. F. Kazennov.

A total of 20 orders were made (and initially 30 were planned). G.K. Zhukov, A.M. Vasilevsky and I.V. Stalin were awarded the award twice, and L.I. Brezhnev was deprived of it posthumously, so the Order of Victory has only 16 gentlemen.
Currently, Russia ranks second in the world in platinum production, and its main source is not the gold-bearing Ural placers, but copper-nickel ores mined in the Norilsk region, where platinum is present in the form of impurities. Other deposits are located in the Khabarovsk Territory (Konder Ridge), Sakha-Yakutia (Inagli, Aldan), Irkutsk Region (Kingash), as well as on the Kola Peninsula. The world leader in platinum production is South Africa (60% of the total); The largest deposit is being developed in the Bushveld massif (Merensky Reef). Other leading suppliers are Zimbabwe, Canada, USA, Colombia.
Every year, platinum is becoming an increasingly popular jewelry material, mainly in the market for engagement rings and diamond jewelry.

In Russia, a metric standard has been approved for it - 990, and for alloys - 850, 900, 950. Moreover, alloys can contain not only platinum group metals (iridium, palladium, rhodium), but also tungsten, copper, cobalt.
Demand for platinum is especially high in Muslim countries, where men are not allowed to wear gold items. But platinum was unknown in the time of Mohammed and since there is no prohibition, it is very often used in men's jewelry and accessories.
In astrology, platinum is considered the metal of Aries, personifying wisdom and mitigating punishment for serious sins. At the same time, it is believed that she cannot stand even the smallest theft. For a kind and generous person, platinum will help protect against illness and achieve success in any endeavor.
In its raw form, platinum is similar to native silver, which is much lighter and softer (scratched with a steel knife), melts under a blowpipe and dissolves in nitric acid. An alloy of iron, chromium and nickel known as “belgica” can be used as an imitation of platinum in jewelry.
Advantages and disadvantages
The benefits of platinum are due to its physical and chemical properties. Among them are hardness, low thermal conductivity, high density, and fire resistance. The metal does not deform when heated and is resistant to corrosion. It is almost impossible to bend or deform.
Platinum jewelry is hypoallergenic, wear-resistant, and durable.
The durability and strength of the precious metal is evidenced by the fact that kilogram and meter standards were made from it at the end of the 18th century.
Against the background of all the listed advantages, there is only one drawback. This is the price. The cost of platinum products is significantly higher than gold ones.
Kinds
Native platinum is classified depending on the content of other components in the ore. There is palladium platinum (40% palladium), nickel (3% nickel), rhodium (5% rhodium), cuproplatinum (10-15% copper), ferruginous (25% nickel and iron each).
Measured, stamped 999 fine ingots are produced from enriched pure platinum. Due to its high cost, Pt jewelry is usually small and monolithic.
Alloys and samples
Platinum alloys, like the pure element, are grayish-white with a characteristic shine.
The list indicates what the ligature consists of. For the alloy, one or more of the following components are taken:
- copper;
- rhodium;
- palladium;
- gold;
- cobalt;
- iridium.
Products made from precious metals, including platinum, are subject to mandatory testing. Placing a state stamp confirms the conformity of the alloy to a particular sample.
According to the metric system adopted in Russia, 850, 900, 950, 999 samples are approved for platinum. The stamp is a rectangle with beveled edges, which depicts the profile of a woman in a kokoshnik and a digital designation of the sample.
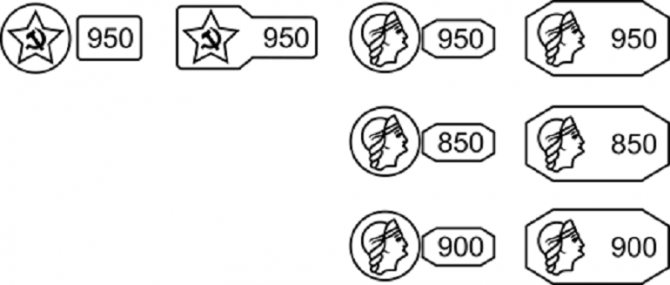
Sample correspondence table
The metric system shows how many units of pure precious metal are in 1000 units of the alloy. The carat standard is based on pure metal as 24 units. The formula for converting a metric sample into a carat sample is: sample × 24/100.
| Metric | Carat |
| 850 | 20 |
| 900 | 22 |
| 950 | 23 |
| 999 | 24 |
Chemical properties

Platinum's chemical properties are similar to palladium, but exhibits greater chemical stability. At room temperature it reacts with aqua regia:
3Pt + 4HNO3 + 18HCl → 3H2[PtCl6] + 4NO↑ + 8H2O
Platinum dissolves slowly in hot concentrated sulfuric acid and liquid bromine. It does not interact with other mineral and organic acids. When heated, it reacts with alkalis and sodium peroxide, halogens (especially in the presence of alkali metal halides):
Pt + 2Cl2 + 2NaCl → Na2[PtCl6]
When heated, platinum reacts with sulfur, selenium, tellurium, carbon and silicon. Like palladium, platinum can dissolve molecular hydrogen, but the volume of absorbed hydrogen and the ability to release it when heated is less in platinum.
When heated, platinum reacts with oxygen to form volatile oxides. The following platinum oxides have been identified: black PtO, brown PtO2, reddish-brown PtO3, as well as Pt2O3 and mixed Pt3O4, in which platinum exhibits oxidation states II and IV.
The hydroxides Pt(OH)2 and Pt(OH)4 are known for platinum. They are obtained by alkaline hydrolysis of the corresponding chloroplatinates, for example:
Na2[PtCl4] + 2NaOH → 4NaCl + Pt(OH)2↓ Na2[PtCl6] + 4NaOH → 6NaCl + Pt(OH)4↓
These hydroxides exhibit amphoteric properties:
Pt(OH)2 + 2NaOH → Na2[Pt(OH)4] Pt(OH)2 + 4HBr → H2[PtBr4] + 2H2O Pt(OH)4 + 2NaOH → Na2[Pt(OH)6] Pt(OH) 4 + 6HBr → H2[PtBr6] + 4H2O
Platinum hexafluoride PtF6 is one of the strongest oxidizing agents among all known chemical compounds, capable of oxidizing oxygen and xenon molecules:
O2 + PtF6 → O2 + [PtF6]−
The compound O2+[PtF6]− (dioxygenyl hexafluoroplatinate(V)) is volatile and decomposes with water into fluoroplatinate(IV), a small amount of hydrated platinum dioxide and oxygen with an admixture of ozone.
Using platinum hexafluoride, in particular, Canadian chemist Neil Bartlett in 1962 obtained the first true chemical compound of xenon, Xe[PtF6].
With the discovery of the interaction between Xe and PtF6 by N. Bartlett, leading to the formation of Xe[PtF6], the chemistry of inert gases began. PtF6 is obtained by fluorinating platinum at 1000 °C under pressure.
Fluoridation of platinum at normal pressure and temperature 350-400 °C gives platinum(IV) fluoride:
Pt + 2F2 → PtF4
Platinum fluorides are hygroscopic and decompose with water.
Platinum tetrachloride with water forms PtCl4 n
H2O, where
n
= 1, 4, 5 and 7. Dissolving PtCl4 in hydrochloric acid produces chloroplatinic acids H[PtCl5] and H2[PtCl6].
Platinum halides such as PtBr4, PtCl2, PtCl2 2PtCl3, PtBr2 and PtI2 have been synthesized.
Platinum is characterized by the formation of complex compounds of the composition [PtX4]2- and [PtX6]2-. While studying platinum complexes, A. Werner formulated the theory of complex compounds and explained the nature of the occurrence of isomers in complex compounds.
Reactivity

Platinum is one of the most inert metals. It is insoluble in acids and alkalis, with the exception of aqua regia. Platinum also reacts directly with bromine, dissolving in it.
When heated, platinum becomes more reactive. It reacts with peroxides, and upon contact with atmospheric oxygen, with alkalis. A thin platinum wire burns in fluorine, releasing a large amount of heat. Reactions with other non-metals (chlorine, sulfur, phosphorus) occur less actively. When heated more strongly, platinum reacts with carbon and silicon, forming solid solutions, similar to the iron group metals.
In its compounds, platinum exhibits almost all oxidation states from 0 to +6, of which +2 and +4 are the most stable. Platinum is characterized by the formation of numerous complex compounds, of which many hundreds are known. Many of them bear the names of the chemists who studied them (salts of Cossus, Magnus, Peirone, Zeise, Chugaev, etc.). A great contribution to the study of such compounds was made by the Russian chemist L. A. Chugaev (1873−1922), the first director of the Institute for the Study of Platinum, created in 1918.
Catalyst
Platinum, especially in a finely dispersed state, is a very active catalyst for many chemical reactions, including those used on an industrial scale. For example, platinum catalyzes the reaction of hydrogen addition to aromatic compounds even at room temperature and atmospheric pressure of hydrogen. Back in 1821, the German chemist I. W. Döbereiner discovered that platinum black promotes the occurrence of a number of chemical reactions; however, the platinum itself did not undergo changes. Thus, platinum black oxidized vapors of wine alcohol (ethanol) to acetic acid already at ordinary temperatures. Two years later, Döbereiner discovered the ability of spongy platinum to ignite hydrogen at room temperature. If a mixture of hydrogen and oxygen (explosive gas) is brought into contact with platinum black or spongy platinum, then at first a relatively calm combustion reaction occurs. But since this reaction is accompanied by the release of a large amount of heat, the platinum sponge becomes hot and the explosive gas explodes. Based on his discovery, Döbereiner designed the “hydrogen flint,” a device that was widely used to produce fire before the invention of matches.
Where can you buy or sell
Demand on the platinum sales market so far exceeds its supply. This is due to the complex extraction from the subsoil. High purity platinum bars and coins can be purchased in banks. They can also be bought back there (usually with a certificate and receipt). Platinum jewelry, which is made by famous jewelry houses, cannot be found in an ordinary store.
You can sell platinum jewelry or scrap at a pawnshop or auction house. The price there will not be the best. The advantage is that the money will be given out immediately. Thrift stores can sell items at a better price, but this may not take a single day. Money will be given to the seller only after the sale of the goods.
You can get a favorable price when selling at auctions, including on the Internet. But an inexperienced seller, in pursuit of profit, can run into scammers.
Expert opinion
Lyudmila Pestereva
Our most experienced gold investor
Ask a Question
I advise you to find collectors. They will evaluate the value of the product not only in grams, but also taking into account its artistic, historical, and jewelry value. In this case, you can sell the jewelry at a price as close as possible to the market price.
How much does 1 gram cost today?
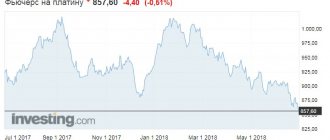
Prices per ounce of precious metal are set twice a day on the London Stock Exchange. The figure is taken as a basis by the central banks of the world's countries, including the Central Bank of the Russian Federation. Below is a live chart and table with current platinum prices.
Platinum | RUB | 1 Gram
USDRUB*PL1!*10000000/311034768 chart provided by TradingView
Platinum | USD | 1 Gram
Chart PL1!*10000000/311034768 courtesy of TradingView
Price per scrap
All items accepted at the pawnshop are assessed as scrap. The cost of platinum scrap is, of course, lower than the market price - by about 15-20%. Some pawn shops value jewelry by category. For high-quality items, a large amount is given per gram.
How to spot a fake
Platinum's silvery luster is similar to silver. Let's figure out how to avoid scammers and distinguish real platinum jewelry from a fake.
The element does not react to alkalis and acids. There is a simple home test using a rotten egg, which contains hydrogen sulfide. By what color the product becomes, you can determine what is in front of you. Silver will turn black, platinum will not.
The old-fashioned method of biting will help determine how soft the metal is in front of you. Platinum alloys are very hard and there are no traces of deformation on them. Of course, not every buyer will risk their teeth. And sellers are not happy with this definition of authenticity.
A distinctive characteristic of platinum is low thermal conductivity. When heated, heat spreads more slowly than with other metals.
I advise you to pay attention to the mark. Images, numbers, and print outlines must be clear and without deformation. If you have doubts about the authenticity of a precious metal, you can contact a specialist - a jeweler or an appraiser at a pawnshop.
Tips for choosing platinum jewelry
It is always better to buy jewelry in large specialized stores that have documents and value their reputation.
Resist the urge to buy platinum on the cheap. This is a fairly expensive metal. It cannot cost slightly more than silver or as much as gold. Due to the high cost of raw materials, platinum jewelry is made small in size. They are cast, not blown.
Before purchasing, we recommend that you check with the seller not only the sample, but also the composition of the ligature. Iridium is a cheaper metal. Ruthenium and cobalt add strength to the alloy.