Being in nature
Gold is everywhere. A cubic kilometer of sea water contains 5 kg of the coveted element, and if you prick your finger and squeeze out a drop of blood, it will contain 0.00025 mg of gold. 10 mg is contained in the human skeleton: if you set out to melt a ring of people, you will only need 300 people. But this gold is in such a dispersed form in the environment that it is unprofitable and often impossible to extract it from there.
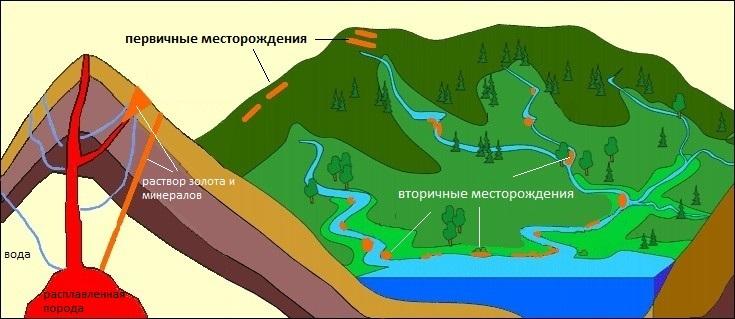
Deposits suitable for gold mining are divided into primary - primary (post-magmatic) and secondary (placer).
Primary deposits
Magma, the melt inside the globe, is rich in the chemical element Au. Gold is found in the upper layers of the mantle and partially in the earth's crust (however, it contains almost the entire periodic table). Magma comes to the surface of the planet, cools and turns into solid rock. The places where it contains enough of the precious element to justify industrial development are primary deposits.
Natural gold is found in the form of nuggets - whole grains of a chemically pure substance. It is often combined with other elements (magma contains almost everything):
- silver;
- copper;
- platinum group metals;
- bismuth and others.
Secondary deposits
Secondary deposits are the result of the destruction of primary ones, the so-called weathering, which happens:
- physical (cause - wind, water, temperature fluctuations);
- chemical (chemical reactions);
- biological (bacteria and other organisms).
A placer of pure gold looks like sand and sometimes drifts along waters many kilometers from the original deposit.
Gold in the biosphere and in medicine
In the biosphere, gold migrates in combination with organic compounds and mechanically in river suspensions. 1 liter of sea and river water contains about 4·10-9 g of gold. In areas of gold deposits, groundwater contains approximately 10-6 g/l gold. It migrates in soils and from there enters plants; some of them concentrate gold, such as horsetails and corn.
In medicine, gold preparations are used in the form of a suspension in oil (domestic drug Krizanil, foreign - myocrisin) or water-soluble drugs (foreign - Sancrizin and Solganal) for injection in the treatment of chronic rheumatoid arthritis, erythematous lupus erythematosus, often in combination with hormonal and other drugs . Gold preparations often cause side effects (fever, irritation of the intestines, kidneys, etc.). Contraindications to the use of gold preparations: severe forms of tuberculosis, diabetes mellitus, diseases of the cardiovascular system, liver, kidneys, blood. Radioactive gold (usually 198Au) is injected into tissues in the form of pins, granules, etc. - for gamma therapy and in the form of colloidal solutions - for beta therapy. It is used in the treatment of tumors, usually in combination with surgical and drug treatment, as well as for diagnostic purposes - in the form of colloidal solutions in the study of the reticuloendothelial system, liver, spleen and other organs.
History of element discovery
In its pure form, gold fell into the hands of man in the 6th century BC. Massive development of African deposits began earlier - around 2000 BC. e., but there were no methods for getting rid of impurities, and gold products of that time are of low standard.
During late antiquity (beginning of our era), alchemy began to spread throughout the world with its desire to transform base chemical elements into noble ones. She was not successful, but thanks to her, modern civilization has mastered many miracles - for example, the technique of extracting chemically pure gold from ore.
The Latin name for gold is Aurum (read as aurum) - “yellow”. It is accepted as international. The symbol of the sun among alchemists looked like a circle with a dot inside, and in modern chemistry it is denoted by the abbreviation Au.
Gold extraction
Sources of gold are sands and ores of placer and primary gold deposits (their gold content is 5-15 g/t), as well as intermediate ones. products (0.5-3 g/t) of lead-zinc, copper, uranium and certain other products. Gold is extracted from placers by gravity. methods using the so-called traps, jigging machines, concentration resins, sluices, decomposition. flushing devices. Gold-bearing sands are mined from the bottom of rivers and lakes and enriched in dredges. When extracting gold from primary ores, a combine is used. schemes including enrichment (gravity, flotation) and metallurgical (leaching, ion-exchange sorption from pulps, cyanidation, less commonly amalgamation) operations. When using cyanidation, the crushed ore or concentrate is treated with NaCN solution with stirring; from cyanide solutions, gold is precipitated with Zn powder, using ion exchange resins or activators. coals. The final products of the scheme are usually gravity concentrate (the so-called gold head) and rough gold. Gold is purified by dissolving it in aqua regia and the afterglow. elect precipitation (for example, using FeSO4), chlorination in a melt or solution (chlorination) and electrolytic. refining in hydrochloric acid solution.
Obtaining gold and refining it. Gold can be extracted from placer deposits by elutriation, based on the large difference in densities of gold and waste rock. This method, which was already used in ancient times, is associated with large losses. It gave way to amalgamation (known already in the 1st century BC and used in America since the 16th century) and cyanidation, which became widespread in America, Africa and Australia in the 1890s. At the end of the 19th - beginning of the 20th centuries. Indigenous deposits became the main source of gold. The gold-bearing rock is first crushed and enriched. Gold is extracted from the resulting concentrate with a solution of potassium or sodium cyanide. Gold is precipitated with zinc from a solution of complex cyanide; At the same time, impurities also fall out. To purify (refin) gold by electrolysis (method of E. Wollwill, 1896), anodes cast from impure gold are suspended in a bath containing a hydrochloric acid solution of AuC13; a sheet of pure gold serves as the cathode. When a current passes, impurities precipitate (anodic sludge, sludge), and gold with a purity of at least 99.99% is deposited on the cathode.
Definition [identification in rocks and minerals]. Gold is detected qualitatively by the formation of colored sediments and solutions. They use connections. gold with Hg2Cl2, H2O2, SnCl2, K4[Fe(CN)6], KI, bsnzidine, 1-naphthylamine, o-toluidine, guaiac resin, complexone III, ascorbic acid, phenylthiourea, dithizone, rhodamine, isoquinoline, etc. You can use sorption on ion-exchange resins, as well as methods of electrophoresis, chromatography (circular thin-layer, precipitation and distribution), and luminescence. Gold is determined quantitatively 1) gravimetrically (in the form of metallic gold), 2) titrimetrically (by the reduction of Au3+ with subsequent titration of the excess reducing agent), 3) photometrically (by the orange color of the bromouraate ion, as well as by the intense color of the compound 3. with decomp. organic reagents), 4) electrochemically, spectral methods, activation methods, atomic absorption. and assay analyses. For pre- Gold concentration uses chemical methods, liquid extraction and chromatography.
How do you get it?
The main methods for producing gold on an industrial scale complement each other - for example, concentrate can be purified from dense impurities by amalgamation.
Flushing
Washing (sizing) is an ancient method of extracting gold sand (size) from secondary deposits. Sand is washed away due to its density: less dense minerals are washed out with water, and the concentrate settles.
Large-scale gold mining is automated: instead of people, washing devices and excavators work. However, the principle of their operation has remained almost unchanged over the past 2000 years.
The concentrate is not pure gold. There are elements that are denser - they settle with sand at the bottom of the washing tank. For final cleaning, other methods, in particular chemical ones, are used.
Amalgamation
This method has also been known since antiquity, but was described in the 16th century. It is possible due to the property of mercury to form alloys (amalgams) with other metals without additional thermal or chemical effects. After getting rid of waste rock fragments, the chemical elements are mechanically separated.
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
Amalgamation is not used everywhere: in a number of countries (since 1988 - in Russia) the use of mercury is prohibited due to the deadly danger of this element to humans.
Cyanidation
The method of extracting a precious element from ore by cyanidation is based on the ability of gold to dissolve in hydrocyanic acid (hydrogen cyanide, HCN) and its salts. The ore is treated with a weak (0.03–0.3%) cyanide solution. The noble metal reacts before other chemical elements, and after the chemical reaction it precipitates from solution.
White gold: composition
White gold refers to gold alloys with palladium or platinum. Sometimes, white gold may contain both of these metals in different proportions. Platinum gold (an alloy of gold and platinum) is considered the most durable, beautiful, but at the same time the most expensive. The percentage of pure gold in the alloy is determined by the breakdown. Very often, white gold jewelry is coated on top with a thin layer of rhodium (rhodium-plated). White gold is an ideal setting for precious stones, especially diamonds.
Gold's place in Mendeleev's periodic table
The element is located in group XI (copper subgroup), period VI of the periodic table of chemical elements.
The atomic number (charge number) of gold is 79. This is the number of protons in the nucleus of an atom, equal to the number of electrons orbiting the nucleus. The atomic mass - the total mass of protons and neutrons (atomic nucleus) - of gold is 196.9665 amu. (atomic mass units). Natural gold exists in the form of the chemically stable isotope 197 Au. All others are unstable and are possible only in a nuclear reactor.

Formula
Gold does not have its own chemical formula, since it exists in the form of monatomic molecules. The electronic configuration of the Au atom is written as [Xe] 4f14 5d10 6s1 and denotes the exact distribution of electrons among the orbitals.
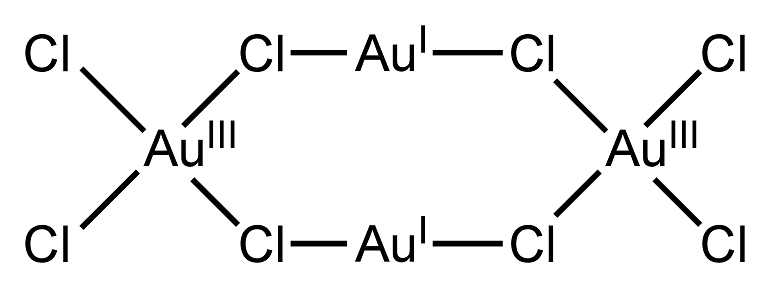
Interaction with acids
Due to its inertness (not absolute, but significant), gold does not dissolve in acids. This allows them to be used for refining (chemical purification of an element from impurities): the alloy is treated with an acid, such as nitric acid, and thus gets rid of the alloy.
But there are exceptions. Pure gold is dissolved by acids:
- selenium;
- cyanide and its salts (cyanides);
- nitrogen mixed with hydrochloric water (regia vodka).
Oxidation states and relationships with halogens
Under natural conditions, Au does not oxidize when exposed to oxygen - this is one of the properties that makes the element precious. When heated, gold reacts with halogens (elements of group XVII): iodine, fluorine, bromine and chlorine, forming iodide, fluoride, bromide and chloride, respectively.
Standard oxidation states are 1 and 3. Fluoride with an oxidation state of +5 has been isolated in laboratory conditions.
Chemical properties of gold and its compounds
The configuration of the outer electrons of the gold atom is 5d10 6s1. In compounds, gold has valences of 1 and 3 (complex compounds in which gold is 2-valent are known).
Gold is characterized by its easy reduction from compounds to metal and the ability to form complexes. The existence of gold oxide, i.e. gold oxide (I) Au2O, is doubtful.
Gold does not interact with non-metals (except halogens). With halogens, gold forms halides, for example 2Au + 3Cl2 = 2AuC13. Gold dissolves in a mixture of hydrochloric and nitric acids, forming chlorauric acid H [AuCl4].
In solutions of sodium cyanide NaCN (or potassium KCN), with simultaneous access to oxygen, gold is converted into sodium cyanoaurate (I) 2Na. This reaction, discovered in 1843 by P.R. Bagration, received practical application only at the end of the 19th century. (see Cyanidation).
Gold(I) chloride AuCl is obtained by heating gold(III) chloride: AuC13 = AuCl + C12.
Gold(III) chloride AuC13 is obtained by the action of chlorine on powder or thin leaves of gold at 200 °C. Red needles of AuCl3 give a brown-red solution of complex acid with water: AuC13 +H2O=H2[AuOC13].
When a solution of AuC13 is deposited with caustic alkali, amphoteric yellow-brown gold(III) hydroxide Au(OH)3 with predominant acidic properties precipitates; that's why it's called golden acid
, and its salts -
aurats
(III). When heated, gold (III) hydroxide turns into gold oxide Au2O3, which above 220° decomposes according to the reaction: 2Au2O3 = 4Au + 3O2.
When gold salts are reduced with tin(II) chloride 2AuC13 + 3SnCl2 = 3SnCl4 + 2Au, a very stable purple colloidal solution of gold is formed ( Cassium purple
); this is used in analysis to detect gold. Quantitative determination of gold is based on its precipitation from aqueous solutions with reducing agents (FeSO4, H2SO3, H2C2O4, etc.) or on the use of fire assay.
Au(OH)3 hydroxide - dark brown crystals; when heated, it dehydrates to form first AuO(OH), and then sesquioxide Au2O3, which decomposes into gold and O2 above 160°C; solubility in water is 2.4.10-12 mol/l at 20°C, in HNO3 solutions - up to 0.38 mol/l at 25°C, in NaOH solutions - up to 8.10-4 mol/l at 25°C. In the latter case, gold is present in the solution in the form of hydroxoaurate ions [Au(OH)4]- (pH 7-13). Au(OH)3 is formed by adding a concentrated solution of alkali or Mg(OH)2 to solutions of H[AuCl4].
Auras are unstable and easily decompose when heated. Alkali metal aurates are highly soluble in water, solubility increases with increasing ionic radius of the cation; Mg, Ca, Sr, Ba, Tl(I) aurates have limited solubility. Aurates form explosive mixtures with some organic substances. It is assumed that when gold hydroxide is exposed to alkali solutions, aurate anions [H2AuO3]-, [HAuO3]2-, [AuO3]3- are formed. See also table. 2.
Other oxygen compounds of gold are unstable and easily form explosive mixtures. The compound Au2O3 with ammonia Au2O3.4NH3 is called “explosive gold”; explodes at 145°C, sometimes at lower temperatures; dissolves without explosion in solutions of alkali metal cyanides.
Gold hemisulfide Au2S - black-brown crystals; DG0arr 29 kJ/mol; poorly soluble in water (p-value product 4.10-69 at 25 °C), sol. in solutions of cyanides and polysulfides of alkali metals. Receive interaction. conc. solution K[Au(CN)2] with H2S from the last. heating to boiling with excess hydrochloric acid. Sesquisulfide Au2S3 - black crystals; decomposes when heated. up to 200 °C; not sol. in salt and sulfuric acid, sol. in HNO3 with the release of elemental gold, KCN solutions, bromine water. Receive interaction. H2S with AuCl3 or complex gold chlorides in anhydrous ether in the cold. Complex compounds containing the anions [AuS3]2-, [AuS2]-, [AuS]-, [Au(SO3)2]3-, [Au(S2O3)2]3- are known.
AuSe monoselenide exists in two crystalline forms. modifications of the monoclinic system. When treating gold hydrochloric acid solutions in the cold with hydrogen selenide, sesquiselenide Au2Se3.H2Se is deposited, which is stable (after drying) in the range of 40-390°C; at 535-650°C it decomposes with the release of elemental gold.
Selenate (IV) Au2(SeO3)3.3H2SeO3 lemon-yellow crystals; not sol. in water, sol. in salt and selenium (when heated) compounds.
Selenate (VI) Au2(SeO4)3 yellow crystals; DH0rev - 954 kJ/mol; not sol. in water, decomposes with hydrochloric acid, sol. in H2SO4, HNO3 and hot conc. H2Se04.
Telluride (hemitelluride) AuTe2 - crystals from brass-yellow to silver-white with metallic. shine; dense 9.3 g/cm, DH0rev - 11 kJ/mol; brittle, Mohs hardness 2.5-3.
AuSCN thiocyanate is colorless. crystals; not sol. in water and org. r-retailers; at 140°C decomposes to metallic. gold and (SCN)n; under the influence of water it forms strong complex anions [Au(SCN)2]- and [Au(SCN)4]- in solutions.
Colloidal gold
When restoring gold in the breakdown. solution of its salts, as well as with electric. By spraying gold in water, colloidal solutions of gold are formed, the color of which depends on the degree of dispersion of the particles, and the intensity of the color on their concentration. Gold particles in a colloidal solution are negatively charged. A hydrophobic gold sol in hydrochloric acid aqueous solution can be represented by the following diagram:
[Au]m is the core of the micelle (the number of atoms m, depending on the conditions, can vary from several hundreds to millions of units); AuCl4- - ions that determine the negative charge of the colloidal gold particle and the potential of the adsorption layer of thickness d0; H+ - counterions that determine the potential of the diffusion layer (electrokinetic potential), of which x ions are located in the blurred part of the double layer with thickness d; n is the number of AuCl4- ions adsorbed on the surface of the micelle core, while n
Measures of gold purity
States control the circulation of precious metals. A century ago, almost every country had its own testing system, but now most have been brought to a common denominator.
British carat system
In the carat system (USA, Canada, Switzerland), the number 24 is accepted as 100%. The mark “18 K” indicates that the jewelry consists of 75% of precious metal, and 25% of something else - for example, copper and palladium .

Metric system
In Russia, the CIS, and Germany, the number on the stamp is the number of ppm (thousandths) of gold in the alloy. 500 ‰ - sample 500, 375 ‰ -375. Only the 1000 sample does not exist - instead there is 999.9. It contains a microscopic amount of impurities and is conventionally considered pure.
Spool system
The spool sampling system operated in the Russian Empire, the RSFSR and the USSR in 1798–1927. It is based on the Russian pound, equal to 96 spools, similar to the carat mathematically, but divides the whole not into 24, but into 96 shares.
Sample correspondence table
Let's look at the three systems in comparison. There is also a lot sample - it essentially repeats the carat sample, but takes 16 units (lot) for one hundred percent. The lot hallmark was used to hallmark silver in Europe before the introduction of the metric system and has no relation to gold.
| Metric system | 999 | 958 | 916 | 900 | 750 | 585 | 500 | 375 |
| Carat sample system | 24 | 23 | 22 | 21,6 | 18 | 14 | 12 | 9 |
| Spool sampling system | 96 | 92 | 88 | 86,4 | 72 | 56 | 48 | 36 |
Gold in the noosphere (application)
Gold is a currency metal that serves as the universal equivalent of money. Gold and its alloys are used for decorative purposes, the manufacture of jewelry, coins, medals, dentures, parts of chemical equipment, electrical contacts and wires, microelectronics products, for cladding pipes in chemical applications. industry, in the production of solders, catalysts, watches, for coloring glass, making nibs for fountain pens, coating metal. surface (in aircraft construction, space technology and other areas). Art. The radioactive isotope 198Au (T1/2 2.967 days) is used to treat tumors in radiotherapy. World production 3. (without the USSR) approx. 1100 t/year (1984). Basic producers - South Africa, USSR, Canada, USA, Brazil, Australia. Some Au(I) preparations are toxic and accumulate in the kidneys, and, to a lesser extent, in the liver, spleen and hypothalamus; the accumulation of gold in the kidneys can lead to kidney disease, as well as dermatitis, stomatitis, and thrombocytopenia. Gold was known to man already in ancient times; it is possible that it was the first metal that humanity began to use for its needs. There is data on the extraction of gold and the manufacture of various types of gold from it. products in Egypt (4100-3900 BC), India, Indochina (2000-1500 BC), etc.
Gold in technology
In technology, gold is used in the form of alloys with other metals, which increases the strength and hardness of gold and allows it to be saved (see Gold alloys). The gold content in alloys used for the manufacture of jewelry, coins, medals, semi-finished products for dental prosthetic production, etc., is expressed by breakdown (see Sample of precious metals, Jewelry alloys); Usually the additive is copper (the so-called alloy). In an alloy with platinum, gold is used in the production of chemically resistant equipment, in an alloy with platinum and silver - in electrical engineering. Gold compounds are used in photography (tinting).
Gold in economics
In conditions of commodity production, gold performs the function of a universal equivalent (money). Expressing the value of all other goods, gold as a universal equivalent acquires a special use value and becomes money. The commodity world singled out gold as money because it has the best physical and chemical properties for a monetary commodity: homogeneity, divisibility, storability, portability (high value for small volume and weight), and easy to process. A significant amount of gold is used to make coins or in the form of bars is stored as the gold reserve of central banks (states). Gold is widely used for industrial consumption (in radio electronics, instrument making and other advanced industries), as well as as a material for the manufacture of jewelry.
The size of gold reserves is an important indicator of the stability of capitalist currencies and the economic potential of individual countries. The purchase and sale of gold for industrial consumption, as well as for private hoarding (accumulation), is carried out on special gold markets (see Gold Markets). The loss of gold from free interstate market circulation caused a reduction in its share in the monetary system of the capitalist world and, above all, in the foreign exchange reserves of capitalist countries (from 89% in 1913 to 71% in 1928, 69% in 1958 and 55% in 1969). An increasingly significant part of the newly mined gold is supplied for hoarding and industrial use (in the modern chemical industry, for rocketry, space technology). Thus, during 1960–70, private hoarding of gold increased by 3.3 times, its industrial and jewelry use by almost 2.3 times, and the gold reserves of capitalist countries remained at almost the same level ($41 billion).
Gold in art
Gold has been used since ancient times in jewelry (jewelry, religious and palace utensils, etc.), as well as for gilding. Due to its softness, malleability, and ability to stretch, gold lends itself to particularly fine processing by embossing, casting, and engraving. Gold is used to create a variety of decorative effects (from the smooth surface of a yellow polished surface with smooth tints of light highlights to complex textured juxtapositions with a rich play of light and shadow), as well as to create the finest filigree. Gold, often colored with impurities of other metals in various colors, is used in combination with precious and semi-precious stones, pearls, enamel, and niello.
How to spot a fake
To make money by passing off products made from base alloys as valuable, scammers resort to tricks: burning silver on fire, combining copper with zinc and tin. Pay attention to:
- Brand - it must meet the standard.
- Price - if it is incredibly low, this is an alarming sign.
- Country of origin - check the jewelry again if it is Turkey, China or the UAE.
There are tips to try something on your teeth in front of the seller or test it chemically by dropping iodine on it. These are effective methods for determining the authenticity of high standards, but they are not always acceptable in society. If the seller makes you doubt so much that you are ready to bite his goods, you should refuse the purchase.
Network news about gold
- Pico-sized gold clusters set catalysis records.
- A completely new form of gold has been created. At very high pressure, gold develops a different structure at the atomic level. The crystal structure of gold is quite stable and resembles an octahedron or cube. Therefore, it is used as a kind of standard in experiments with high pressure. When scientists quickly compressed gold at very high temperatures, it formed what is called a body-centered cubic lattice (BCC), in which the atoms are “packed” less efficiently.
Amalgamation
Mercury, when interacting with gold, due to diffusion, is able to attract particles of the precious metal without dissolving it and forming a liquid alloy, which is called amalgam. The property of mercury to only wet gold, taking it into its shell without entering into a chemical reaction, was taken as the basis for the amalgatation method. The moistened fractional rock is mixed with mercury and left for several hours to amalgamate, and then the remaining slag is removed. By heating the amalgam, mercury is separated from gold, the resulting sludge is washed and used for further processing. The greatest effect is achieved when the gold particles are as free from oils and deposits as possible, so before amalgation it is advisable to carry out chemical and mechanical cleaning.
Regeneration
Gold plating of various jewelry and household items is often used in the jewelry, watch industries and medicine; gold processing of medical equipment. Today, due to the intensive growth in the production of electronic equipment, manufacturers are placing increased demands on the reliability of finished equipment. Taking this fact into account, many parts are coated with gold and therefore the volume of electroplating and chemical coatings has increased many times.
Therefore, in many jewelry factories and workshops, increased attention is paid to the regeneration (removal) of gold from waste solutions and electrolytes. Also, due to the increase in sales of scrap radio-technical parts, the extraction of precious metals by regeneration is in increased demand.
← Gold refining↑ Information about goldGold smelting →