The story of Nicolas Flamel
The story of Nicolas Flamel, a book copyist from Paris, is still considered mysterious. This man tried for a long time to get gold from mercury. There is a legend that back in the 14th century he unraveled a mystery that had interested people for centuries: is it possible to artificially produce a precious metal. It all started with the fact that an ancient manuscript with incomprehensible signs and symbols fell into the hands of this man. Nicolas tried to decipher this text for 20 years, but all efforts were unsuccessful. None of the experts in ancient languages to whom Flamel turned could help him.
To unravel the mystery of the manuscript, I had to travel outside of France. And only in Spain, after two years of searching for the right person, did luck smile on him. Here he met a real expert in the ancient Jewish language. The scientist, having learned about the manuscript, immediately went with Nicolas to Paris, since the copyist did not risk taking the texts with him. But the scientist was unable to reach France; on the way he fell ill and died. But still, he managed to tell Flamel something.
Armed with the knowledge gained, Flamel began deciphering the manuscript. His labors were not in vain; in January 1382, Nicolas was able to obtain silver from mercury, and soon his experiments with gold were crowned with success. Perhaps this is just a legend. But the fact is certain that the modest scribe became the owner of a huge fortune in a short period of time. After his death, many seekers searched his house for gold, but no one was able to find anything. There is still no evidence that Flamel knew how to make gold from mercury.
First "successes"
The alchemist Gobmerg was able to obtain gold by melting silver with antimony ore. There was not much precious metal at the output. But the alchemist believed that the secret of transforming metals had been revealed to him. True, with an accurate analysis, it simply turned out that a certain percentage of gold was there from the very beginning.
The pharmacist Kappel was able to achieve a similar result in 1783 - he obtained the precious metal from silver using arsenic. This may be solely due to the precipitation of lead iodide. And the gold, as you probably guessed, was already in the ore.
One more example
Many years have passed since the discoveries of Nicolas Flamel. But the question of how to get gold from mercury remained open. Only at the end of the 19th century, the chemist Stefan Emmens announced to the whole world that he had managed to obtain a substance that could be called a precious metal.
The chemist called the substance obtained experimentally “argentaurum”, and it was made from silver, with the participation of mercury. Researchers from the USA carefully tested that substance and bought it back at the price of gold. These were three test bars. The scientist himself stated at the time that he was not going to disclose the technology and put gold into mass production, as this could have a bad effect on the economy not only of the United States, but of the whole world. But still, Emmens agreed to demonstrate the experience in Paris, at the World Exhibition. Shortly before the performance, the chemist disappeared without a trace. Most likely, his discovery was considered too dangerous.

Introductory information
Papyrus was found in the tomb of the Egyptian city of Thebes at the beginning of the last century. It contained 111 recipes, among which were those that considered the possibility of obtaining silver and gold. But, alas, this was aimed at creating fakes or coating other, less expensive objects with precious metals.
You may be interested in: Naphthenic acid - features, properties, application and formula
Nevertheless, this document showed that alchemy, even in ancient centuries, captured the minds of people thirsting for easy money. Spreading through the Egyptians and Greeks, it was able to gradually take over all of Europe. The greatest practical dawn came in the Middle Ages. At that time, not only scientists, but also state and church officials were interested in alchemy. Thus, in almost every imperial palace one could find “specialized” people who were supposed to receive gold in order to improve the state of the treasury. It was widely believed that this could be done with the help of the philosopher's stone.
What is mercury
Mercury is called "living silver". This silver-colored metal remains in a liquid state at temperatures down to -39 ° C and at the same time has extraordinary mobility. At temperatures below -39 ° C it becomes a solid metal.
Mercury is odorless and tasteless, and evaporates easily at room temperature. The vapors of this substance are very dangerous to human health. Therefore, in domestic conditions, a broken thermometer can cause severe poisoning.
Pure mercury is extracted from an ore called cinnabar. This mineral substance is specially heated to high temperatures so that the mercury can evaporate, and then it is condensed. The densities of mercury and gold are 13,600 kg/m3 and 19,300 kg/m3, respectively.
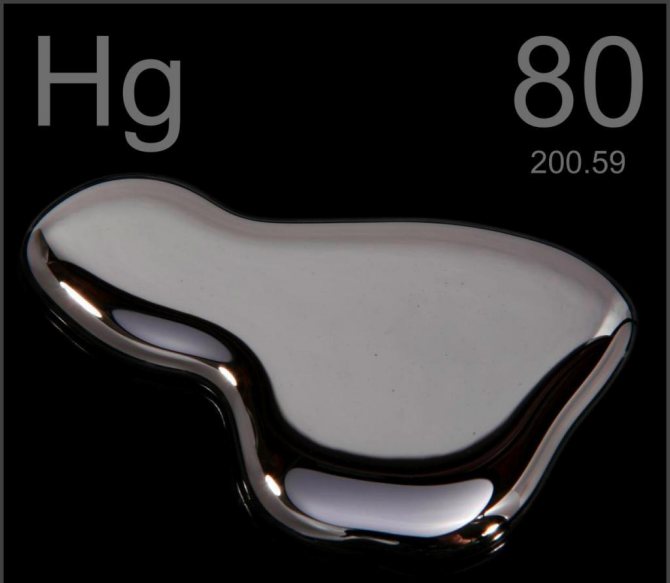
Liquid mercury has the ability to roll into a ball, and it also has a pronounced ability to wet certain metals. A mercury ball can attract gold dust and absorb it into its mass. Ultimately, when the ball can no longer absorb gold particles, it will begin to crumble as a single mass.
Amalgamation method
This method of extracting gold from mercury is considered one of the most ancient. It is very harmful to health, therefore it is banned in the Russian Federation, but in many countries it is still used.
Amalgamation is the process of mixing mercury and a metal such as gold. Mercury balls do not dissolve the metal, but only moisten it, absorbing it. Subsequently, using various methods, such as evaporation, pure gold is obtained.
This method is used if washing black sand and grains of gold smaller than one millimeter does not help.
Paracelsus (1493-1541): predicted homeopathy
Paracelsus is known to the vast majority of readers as a physician and the forerunner of pharmacology, but he also practiced medical alchemy. This scientist was an innovator. the ideas of Aristotle and Galen outdated and demonstrably burned their books. For a long time he tried to create a philosopher's stone, but not for making gold, but for the sake of obtaining the elixir of immortality and miraculous medicines.
Our medicine owes a lot to Paracelsus. It was he who predicted modern homeopathy, began to consider the processes occurring in the human body from the point of view of chemistry and use minerals in the manufacture of medicines. The doctor also laughed at those who considered epilepsy to be possessed by the devil.
He said that he nevertheless created the philosopher's stone and would live forever, but for some reason he died at the age of 48, falling from a great height.
Physician and alchemist Paracelsus. Source: Wikimedia
Prohibited in Russia
In Russia, gold mining from mercury has been banned since 1988. At that time, the USSR Committee for Drugs and Metals issued an order “On stopping the use of mercury (amalgamation) in technological processes during the enrichment of gold ores and sands.” Before the publication of this document, the method using mercury was widespread in gold mining in the USSR. And the consumption of “liquid metal” in the gold mining industry reached hundreds of tons per year. At the same time, a huge amount of mercury entered the environment. To this day, gold miners find mercury waste in places where factories once stood.
Alchemical gold. Are scientists hiding medieval recipes?
As we know, from time immemorial gold has been a measure of wealth, success and, ultimately, power. It is not surprising that court alchemists existed at the courts of the largest monarchs in Europe. It is now believed that all their attempts to obtain gold from base metals were in vain. However, some historical chronicles claim the opposite...

Alchemists of His Imperial Majesty
The most interesting thing is that among the court alchemists one could meet quite venerable scientists. In particular, in Russia this position was officially held by the founder of Moscow University, Mikhail Vasilyevich Lomonosov. In this regard, the statement that the alchemists of the past were entirely charlatans and adventurers is fundamentally incorrect.
The list of Russian alchemists who worked at the court of Russian monarchs alone is impressive. During the time of Ivan the Terrible, the alchemist Ignazio Dagi conducted his research in Moscow. During his experiments, this learned man was able to obtain new ways of isolating silver from ore, which greatly delighted the king. Thanks to this invention, silver mines began to bring twice as much money to the state treasury.
Of course, this is not yet an opportunity to brew gold from lead and mercury, but the positive effect is still evident. Subsequently, under Peter I, the court alchemist Heinrich Apfelbaum became famous for his scientific research. As a result of his experiments, the much-warred country received an improved formula for gunpowder. It should be noted that the position of court alchemist existed in the Russian Empire quite officially until the October Revolution.
Experiments in Bukhara
One of the last major alchemical projects in our country before 1917 was an attempt to obtain artificial diamonds. It was headed by the last state alchemist of the Russian Empire, Count A.V. Tolstoy. Returning to his homeland after studying in Germany, Tolstoy tried to get gold from lead. Moreover, as the count claimed, he had no doubts about how to do this. The question was: where can I get enough energy for this? To solve this problem, Tolstoy built a huge parabolic mirror near Bukhara. A ray of sun focused with it, according to people who participated in the experiments, melted refractory metals.
They tried to get gold from this installation in 1912. The most interesting thing is that the results of this experiment were never officially published. However, already in the Soviet years, Academician of the USSR Academy of Sciences Aron Holstein, who helped Tolstoy in his experiments, claimed that gold had been obtained. True, the academician made a reservation that a tiny amount of precious metal was obtained from lead and it was not clear whether it was an alchemical transmutation or grains of gold that were originally in the lead. It should be noted that these experiments, as well as the construction of a special laboratory, were carried out with the financial resources of Prince P. A. Oldenburg, brother-in-law of Nicholas II.
Read: Genghis Khan's revenge - everything, as always, is because of a woman?
It is noteworthy that after completing work on converting lead into gold, Tolstoy received the title of life alchemist, which may speak in favor of the success of his experiments. Later, the count quite successfully carried out work on converting graphite into diamond, achieving serious success in this field. In 1915, the diamond created in this way, weighing 22 carats after cutting, was recognized as having jewelry value. In honor of the emperor’s daughter, he was given his own name “Anastasia”.
It should be noted that these alchemical experiments brought tangible financial dividends to the Russian crown. Before the outbreak of World War I, the count produced about fifty million dollars worth of diamonds for the state treasury. But diamonds are not gold - the main long-standing dream of all alchemists in the world. However, if difficulties arose with obtaining it in Russia, then in Europe, they seem to have been successfully resolved back in the Middle Ages.
Isaac Newton: gold is more important than physics
As surprising as it may sound, one of the most famous European alchemists of the Middle Ages was the founder of modern physics, Isaac Newton. In our country, this scientist is mainly known for the physical laws he discovered. However, it is little known that Newton devoted more than thirty years of his life to alchemical research. The famous chemist looked for recipes for obtaining gold from base metals in the sacred texts of ancient civilizations.
At the same time, Isaac Newton was not only a famous scientist and alchemist, but also the manager of the British Mint. Working in this responsible and very prestigious position, Newton introduced and achieved the adoption in the English Parliament of a law prohibiting the disclosure of methods for transmuting metals. Simply put, the famous physicist achieved a legislative ban on attempts to obtain gold by alchemical means. According to him, these experiments could radically undermine the stable price of gold on the market. At the same time, a completely logical question arises: if it is impossible to obtain gold by alchemical means, why introduce a ban on such experiments? It can be assumed that Isaac Newton found or knew a way to “cook” gold and was afraid of information leakage.
Gold from a nuclear reactor
Today it is difficult to reliably say what motivated the famous physicist when he initiated the law banning alchemical experiments in England, but his followers in the 20th century actually managed to obtain gold from mercury! This amazing event happened in the 1940s in the USA. Continuing the experiments of their medieval predecessors, American nuclear physicists tried to bombard mercury and platinum adjacent to gold in D.I. Mendeleev’s periodic table with fast neutrons.
Read: The death of Boris and Gleb - the secrets of the Rurikovichs
The experiments were successful. In the spring of 1941, several scientific publications in the United States published articles talking about the successful production of gold. A. Scherr and K. Bainbridge from Harvard University reported about the unique experiment to the scientific community. According to scientists, they obtained three isotopes with mass numbers 198, 199 and 200, while the natural isotope of gold has a mass number of 197. Soon nuclear scientists were able to isolate gold. However, after a few days it turned back into mercury. This happened because the isotopes of gold obtained artificially were extremely unstable. Only six years later, in 1947, the Americans were able to obtain 35 micrograms of gold from 100 mg of mercury from isotopes 196 and 199 during experiments with slow neutrons in a nuclear reactor. But the useful yield of noble metal from mercury-196 was 24%.
Dangerous discovery
It seemed that the extraction of gold from mercury by US physicists was going to become a world sensation. Alas, this did not happen. Fearing an unhealthy reaction from society, they decided not to advertise the discovery. Only two years later did newspapermen from popular tabloids learn about the experiments carried out. Several high-profile publications appeared and, as a result, panic on the stock exchange and the collapse of gold price quotes in 1949. It got to the point that the US authorities, in order to reassure the population of the country, were forced to initiate a number of publications in scientific journals, which stated that producing gold in a nuclear reactor was indeed possible, but expensive and unprofitable.
Nevertheless, as evidence of the realization of the centuries-old dream of alchemists around the world, a piece of gold “welded” in a nuclear reactor is still located at the Chicago Institute of Science and Industry. In fact, speaking in the language of numbers, according to scientists, from 50 kg of mercury you can get 74 grams of the isotope mercury-196. During further transmutation in a nuclear reactor, the isotope gold-197 can be removed using neutron bombardment of the released isotope. However, these manipulations require a lot of time and a lot of energy, which makes this method of obtaining gold unprofitable.
Mystery of the century
Surprisingly, American nuclear physicists were able to isolate gold from mercury, from the very metal that the alchemists of the Middle Ages spoke about. But how could they know that mercury was the metal with which it was necessary to conduct experiments? Obviously, scientists of the past had some secret of how to obtain gold from mercury without the help of a nuclear reactor, which they, of course, did not have.
The fact that such experiments took place can be evidenced by the fact that many coins and gold items allegedly obtained through alchemical experiments are kept in European museums.
Read: Emperor Alexander III wanted to seize a colony in Africa with the help of the Cossacks?
Moreover, many of these exhibits have corresponding signatures so that others have no doubts about how this or that gold coin was obtained. The main part of them has approximately the following content: “A miraculous transformation performed in Prague on January 16, 1648 in the presence of His Royal Majesty Ferdinand III.” In the capital of Austria, Vienna, anyone can see a gold bar, according to legend, obtained by the alchemist I. K. Richtausen in front of the king.
The fact of the event is confirmed by the corresponding signature: “Received in Prague on January 15, 1658 in the presence of His Holy Imperial Majesty Ferdinand III.”
Moreover, medieval alchemists had a fair sense of humor. A museum in Prague houses a half silver, half gold coin obtained by the Czech alchemist Wenzel Seiler. For his unusual experiment, the scientist was awarded by Leopold I the title of nobility and the right to create gold coins from lead.
No less surprising is the fact that after the death of the Holy Roman Emperor and part-time alchemist Rudolf II, 84 centners of gold were found in his storeroom. Did medieval alchemists really know the secret of transforming lead and mercury into gold? If this is so, then it is unclear why they did not produce gold in unlimited quantities. There really seemed to be a secret.
This can be indirectly judged by the story that happened to Lomonosov, who, as already mentioned, was a court alchemist. As is known, in his youth the scientist studied in Germany with local metallurgists. During these years, encrypted texts of alchemical content fell into his hands. However, according to Lomonosov himself, left in his diaries, Christian Wolf, a professor at the University of Marberg, recommended that he not decipher these texts. Later, information appeared that shortly before his death, Lomonosov burned some sheets of paper with some mysterious formulas and magical signs. It is quite obvious that the alchemists of the Middle Ages knew a way to transmute lead and mercury into gold. However, it was obviously very complex and energy-intensive, which is why gold was not produced on an industrial scale.
But scientists are a jealous people. To prevent competitors from getting the secret and to prevent a collapse in gold prices, the alchemical recipe was encrypted, and only a select few were initiated into the secret...
You might be interested in:
- Alchemy - what were the alchemists looking for?
- The Philosopher's Stone and other secrets of medieval alchemists
- Alchemist from the Third Reich
- The elixir of eternal youth was invented in Russia
- In search of the elixir of immortality
- The sword of Prince Svyatoslav was found
Dangerous

Mercury vapor is very poisonous. Therefore, when working with this metal, it is necessary to observe safety precautions. Vapors should not be inhaled as this may cause serious poisoning. In addition, mercury and its compounds should not come into contact with the skin. When interacting with mercury, it is best to wear safety glasses and gloves, and the procedure for extracting gold using mercury should be performed in fresh air. In this case, it is advisable to make sure that the wind blows in the opposite direction from you and residential buildings.
Interaction with acid is as dangerous as interaction with mercury. For the reaction of gold and mercury, or rather to remove excess “liquid metal” during the amalgamation process, nitric acid is used. Therefore, you need to be especially careful when performing manipulations, take care of your skin and eyes, and avoid inhaling acid fumes. To wash off any acid that gets on your skin, you can use clean water.
There is one more rule: when making a solution, it is best to pour the acid into water, and not vice versa. This will help avoid splashing. You can neutralize the effect of acid using soda.
When working with acid, clean water should always be on hand to quickly dilute the acid in case of contact with skin or equipment.
Acid getting on the body causes burns if it is not washed off immediately. Even if it gets on clothing, it will most likely penetrate the skin. In this case, you need to take off your clothes and wash the burned area. It is also recommended to wear a special mask when working with acid; this will help prevent burning your lungs when inhaling the vapors.
Preparation
In order for mercury to absorb gold, it is necessary to clean it of foreign impurities, since they will interfere with the amalgamation process. Sometimes the precious metal is coated with a film of oil or other impurities. A ten percent nitric acid solution is used for cleaning. The washed concentrate is poured into it.
During this process, a reaction may occur that releases gas. It is necessary to wait until all signs of the reaction cease and then rinse the concentrate with clean water, thus washing off the acid.

The process itself
The entire process is carried out in a steel or plastic rinsing tray. The amount of mercury must be equal to the amount of gold in the concentrate. You don’t need too much mercury, since in this case it will be inconvenient to work with. It is better to initially pour less and gradually add more. There should also be a small amount of water in the tray during the process:
- We take the tray in our hands and make circular movements until all the gold that was visible is combined with a ball of mercury. Black sand does not absorb mercury.
- After this, wash off the black sand into a basin of water.
- If during this process a small amount of amalgam leaks into the basin, do not worry. It can always be easily removed from a basin of water.
- We mean that mercury does not capture platinum. Therefore, we carefully watch during the final rinsing.
- If during the process the mercury ball begins to separate, add a little more mercury so that all the gold contained in the sand is absorbed.
- A mercury bead that is completely filled with gold will be 50% mercury and 50% precious metal.

How to make gold from mercury
Once all the gold has been amalgamated and the amalgam itself has been separated from the sand, you can begin to remove the excess mercury. This can be done using extrusion. To do this, take thin and damp suede or any other dense material. All excess mercury must pass through the pores of the fabric. It is best to fill the container that will be located under the material with water. This way, excess mercury will not be splashed, and it can be easily collected in the future. This process is best done while wearing rubber gloves to prevent the mercury from being absorbed into the skin.
The mercury that has been squeezed out of the amalgam will contain a small amount of gold. These residues will help collect more precious metal in future amalgamation processes.
Once all excess mercury compounds have been removed from the ball, you can begin to separate the gold from the mercury. To do this, you can use one of two methods: evaporation, by heating the amalgam, or dissolving mercury in nitric acid.
Mercury processing

The most suitable for manipulation are materials with mass numbers of 196 and 199. Thus, from 100 grams of mercury you can expect approximately 35 micrograms of gold. It is easy to guess that due to the high cost of nuclear transformations, the price turned out to be much higher than the market price. Therefore, this method has not gained popularity.
Obtaining a stable isotope (gold-197) is theoretically possible on an industrial scale from mercury-197. But such a chemical element does not exist in nature. Although you can also pay attention to thallium-201. The truth here is that the problem is of a different nature - this element does not have alpha decay. Therefore, it is more urgent to obtain the mercury-197 isotope.
It can be obtained from thallium-197 or lead-197. It would seem, at first glance, that the second option is much easier. But even in this way, it is more difficult to obtain gold from lead, because these materials do not exist in nature and must be synthesized through nuclear transformations. That is, it is possible to make precious metal, but it is very difficult and expensive. And so the considered option is the most realistic answer to how to make gold from lead.
Evaporation
Mercury evaporates at a temperature of 357 degrees, which is found in the upper parts of the flame of gas burners. Since mercury vapor is highly toxic and can cause fatal poisoning, this procedure should be carried out outdoors. In this case, the wind should not blow towards the person. Mercury can be present on gold in the form of a thin, invisible film, so if the metal appears clean, do not assume that there is no mercury on it.
You can use a steel tray or frying pan for this process. Aluminum containers are not very suitable for evaporation, as aluminum can react with mercury.
Before heating the amalgam bead in the tray, you need to remove as much mercury as possible from it using the method indicated above. At first, the ball is heated slowly, gradually increasing the temperature. If gold contains small amounts of mercury compounds, you don't have to worry about them spattering.
Bernardo of Treviso (1406-1490): received the philosopher's stone
The count of a small Italian border state, subordinate to Venice, began to study alchemy at the age of 14, at the suggestion of his father. He evaporated crystals of the philosopher's stone, tried to isolate primary matter from chicken eggs, and spent all his fortune in search of truth.
He wandered, continued his search, and finally settled on the Greek island of Rhodes. It was there, at the age of 82, three years before his death, that the secret was revealed to Bernardo and he received the philosopher's stone.
He also discovered the secret of a serene life, which turned out to be very simple: a person should be content with what he has.
Interesting history Movies and TV series Science Our heroes
Acid method
Nitric acid is often used to separate gold from mercury after the amalgamation process. By reacting with mercury, it dissolves it without having any effect on the gold. Before you begin, you need to make sure that the amalgam does not contain excess mercury and black sand impurities:
- Place the mercury ball in a glass jar.
- Pour in an acid solution in a ratio of 6:1, stronger if possible.
- We wait until the chemical reaction takes place.
- We rinse the jar well with clean water and pour it into a separate container.
- If the gold has not taken its natural form of flakes and powder and remains of mercury are visible, drain the water and pour in another portion of nitric acid. In case of another failure, we make a stronger solution.
As a rule, with a small amount of mercury, cleansing occurs the first time. If there is a lot of mercury, all steps will need to be completed several times.
If using this method a large amount of “liquid metal” is dissolved and there is a desire to preserve it, you can use the following method:
- Drain the acid after the process into a separate jar. It will contain mercury that has been removed from the amalgam.
- Place aluminum foil in the jar.
- The acid will react with the aluminum and precipitate mercury to the bottom of the jar.
- We drain the acid from the container, neutralizing it with baking soda, until the gas evolution stops.

Use of mercury in modern industry
Mercury has a number of unique properties, which allows it to be valuable in almost any production. Here is a list of industries where this metal is used:
- Chemical industry. The largest amount of mercury is used in the production of chlorine components; it acts as a cathode in the production of sodium and chlorine. Mercury is also used as a catalyst in the formation of certain compounds.
- In nuclear energy, this metal is used to dissolve uranium. Mercury also helps with the thermochemical reaction of splitting water into oxygen and hydrogen.
- Metallurgical industry: here, with the participation of mercury, a number of important alloys are formed, necessary for engraving, lithography and electroplating. This area also appreciates the ability of mercury to absorb many metals, forming amalgams.
- Mercury compounds are often used in the production of precious metals.
- Electrical production: fluorescent, quartz and fluorescent lamps, current rectifiers using a liquid mercury cathode, batteries. Dry batteries, which use mercury in their production, power modern hearing aids.
- Heavy engineering industry: various vacuum installations, modern mercury diffusion pumps, heavily loaded hydrodynamic bearings, a large amount of mercury in liquid form is found in mercury-steam turbines.
- Even in astronomy this metal has found its use. In this field, the "horizon" device is often used, in which mercury is necessary to create the flawlessly mirror-like surface necessary for observing celestial objects.
- In the mining industry it is used to extract gold.
- Instrumentation: used for the manufacture of instrumentation equipment, barometers, thermometers; necessary for assembling washing machines, air conditioners and refrigerators.
- In petroleum refining, metal in the form of vapor is used to regulate the temperature required for refining.
- Mercury is widely used in medicine: it is involved in the creation of diuretics, antiparasitics and antiseptics. And in dentistry it helps in the manufacture of dentures and fillings from an amalgam of certain metals.
- Mercury compounds are also used in photography, leather processing, fabric dyeing, pyrotechnics, and porcelain production.
- In the military industry, liquid metal is used to make explosives, the so-called “mercury fulminate.” This substance is used in the detonators of shells and grenades.
- Shipbuilding. The paint used here contains mercury. It is used to treat the surfaces of ships under water. This paint, when interacting with sea chlorine, forms a film that kills harmful bacteria.
- In agriculture, mercury compounds are used as herbicides.
We learned about the purpose of using mercury in various industries.