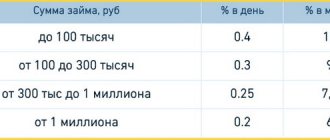
Good afternoon, dear reader! Let's talk today about what gold refining is, why there are so many refining technologies and what benefits it brings.
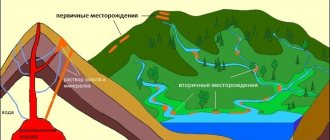
Russia's gold reserve at the beginning of autumn 2021 amounted to 1968 tons. All this is very high standard gold, almost pure - this does not occur in nature. The main product of mining is not large nuggets, but quartz veins, alloys and sand washed out by water (secondary deposits). In order for grains of precious metal to turn into an ingot, they must go through a whole processing cycle.
What is refining and why is it needed?
Refining is the process of deep purification of metal from impurities, that is, increasing the sample. His techniques are based on differences in the chemical and physical properties of the metals present in the alloy. Gold is an inert metal (it almost does not react with acids and does not form oxides), and this simplifies the process of isolating it from the semi-finished product.
Alloys obtained from different sources and having different degrees of purity are refined:
- gold-containing mixtures of substances mined in nature;
- gold scrap (technical, jewelry, household);
- sludge remaining after purification of copper, silver, zinc (in our case, after electrolysis);
- lead production waste - “silver” (zinc) foam with impurities of noble metals;
- gold-containing electronic waste (chips, memory cards, transistors).
The largest share of raw materials entering refining processing is the so-called “rough gold” - products of enterprises engaged in gold mining and primary ore processing. The rough metal is represented mainly by spot gold (placer gold) and sludge, less often by natural ingots.
Legal aspects
The state controls transactions with precious metals, stones and pearls, and their illegal trafficking is regarded as a threat to economic security. Article 191 of the Criminal Code of the Russian Federation provides for citizens to be held liable for illegal actions:
- buying or selling;
- storage;
- transportation (carriage, forwarding).
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
The exception is household items, scrap and jewelry. Criminal liability arises only if the size of the transaction or the volume of stored precious metal or semi-finished product is large (the transaction amount or the cost of the precious metal is 1.5 million rubles or more). Otherwise, the violator will only face administrative punishment.
Where can the procedure be performed?
- Gold refining is carried out by specialized enterprises. In Russia, these are Uralelectromed JSC, Kolyma Refinery and several other organizations. The main method of purifying metal from impurities on an industrial scale is electrolytic (separation of the melt into its component parts by passing an electric current through it).
- There are laboratories with the necessary equipment and hood.
- Some refining techniques can be reproduced at home if you take care of the materials and tools.
Industry development prospects
Not only jewelry workshops and home craftsmen have a need for high-purity precious metals. For the latter, by the way, it is important not to forget that when refining gold and selling the results of chemical transformations, disagreements may arise with the current legislation, so it is so important not to overstep the boundaries of what is permitted...

The St. Petersburg Mint, opened in 1724 on the initiative and with the active participation of Peter I, was the first refining enterprise in Russia at that time, and today this figure is close to ten. Most of the plants work with gold produced from mines, and their clients include the largest domestic gold miners. Essential criteria for selecting a refinery are:

- level of production discipline;
- competent document flow;
- short deadlines for completing work;
- high percentage of gold recovery;
- affordability of prices.
To do this, enterprises need competent long-term strategies that involve reducing the cost of refining. This also includes expanding the scope of the raw material base, introducing innovative energy-saving technologies, and modernizing backward areas. This will not only make it possible to meet the demands of the most demanding customers, but also increase the country’s gold and foreign exchange reserves.
Gold refining methods
Methods for purifying precious metals are divided into:
- chemical (based on the interaction of substances, there are dry and wet);
- electrochemical (electrolysis).
Dry
When talking about the dry method of gold purification, they mean the Miller method. It is used only in industrial conditions due to the toxicity and corrosiveness of chlorine and its compounds, which are released in excess during the reaction.

The essence of the method: chlorine gas is passed through the crushed mass of the processed substance. Compounds of base metals with chlorine are volatile and are removed from the alloy, increasing the gold purity.
Miller's method is effective due to the fact that noble metals react with chlorine last (zinc and iron are removed first, gold and platinum are removed last). Its advantages:
- it is inexpensive;
- does not require large areas to accommodate equipment;
- short - takes several hours;
- removes almost all alloy, increasing the gold content in the alloy to 99.5–99.9%.
The refining process takes place in a crucible (a refractory melting tank), into which chlorine enters through a pipe. Excess components are removed from the mixture, and silver chloride rises to the surface of the alloy - this makes it possible to further separate the noble metals from each other. Miller's method helps to obtain gold from both a multicomponent alloy and an alloy with silver.
Wet
It is convenient to separate the noble metal from the alloy by dissolving either the metal itself or impurities.
The most popular refining method involves reacting scrap with aqua regia (a mixture of nitric and hydrochloric acids - one of the few compounds that dissolves gold). The solution is evaporated and the gold is precipitated using iron sulfate (also suitable for reduction from chloride), oxalic acid, sodium pyrosulfite or hydrazine.
If the reduction reaction is carried out correctly, the loss of pure substance at the output will be minimal, and the sample will reach 999.

There is a way to dissolve gold using Lugol's solution - a combination of potassium and iodine.
Quarting is also used (from the Latin quarta - fourth) - alloying gold in a ratio of 1:3 with another metal (brass, zinc, copper), which is subsequently dissolved in nitric acid. Impurities will not dissolve qualitatively if their content is less than ¾ of the volume, so the semi-finished product is quartted. Theoretically, you can try this at home, but be aware that when dissolved, it releases very poisonous nitric oxide.
If you have gold in solution, it can be easily refined with stannous chloride powder. A day after the start of the process, the gold will settle to the bottom of the container in which you left it. The good thing about the method with tin chloride is that when using it, the operator’s body is not endangered, as when working with volatile chlorides or acid.
Electrolytic
Electrolysis involves the separation of the constituent components of the alloy on the electrodes as a result of the passage of electric current through the electrolyte. During refining, the anode (electrode with a positive potential) is a gold-containing alloy, and the cathode (electrode with a negative potential) is thin rolled gold (999) tin. An electrolyte is a solution of gold chloride and an acid that dissolves the anode.

The initial anode sample is a minimum of 900. The process takes place in small porcelain baths (~ 25 l) installed on water baths to maintain a temperature of 50–60 ° C. Gold particles settle in layers on the cathode. After the process is completed, the anode sludge is sent for further processing: the silver is separated and melted into anodes for silver electrolysis.
What methods of affining can be carried out at home?
Of course, you cannot conduct an experiment with chlorine at home. But you can try to extract gold from scrap or computer waste using tin chloride and acids. Skilled alchemists can use the electrolytic method. But don't forget about safety precautions!
Electrolytic gold refining
Refining gold by electrolysis allows you to obtain high-purity metal.
Anodes are cast from a refined alloy containing silver, platinum metals and some base metals as impurities. The electrolyte is an aqueous solution of chloroauric acid with the addition of hydrochloric acid:
Au (cathode) | HAuCl4, HCl, H2O, impurities |Au with impurities (anode).
Hydrochloric acid is strong and completely dissociates into ions:
HAuCl4 ⇄ Н⁺ + АuСl⁻4.
In turn, the AuCl⁻4 anions partially dissociate to form Au³⁺ cations:
АuСl⁻4 ⇄ Au3+ + 4Сl⁻.
In an aqueous solution, AuCl⁻4 ions can undergo hydrolysis:
AuCl⁻4 + H2O ⇄ [AuCl3 (OH)]⁻ + H⁺ + Cl⁻.
However, in an acidic solution, hydrolysis practically does not occur.
Thus, we can assume that gold in the electrolyte is in the form of the AuCl⁻4 anion
The main cathodic process during electrolytic refining of gold is the reduction of AuCl⁻4 anions to metallic gold:
AuCl⁻4+ 3е ⇄ Au + 4Сl⁻
The standard potential of this process is +0.99 V, so the competing process of hydrogen reduction is practically excluded.
At the anode, the alloy being refined dissolves with the transition of gold into solution:
Au + 4Сl⁻ -3е → АuCl⁻4.
Since the standard potentials of chlorine and oxygen are much more electropositive than the potential of gold:
2Сl⁻ - 2е → Сl2 (gas), φ0 = + 1.36V,
2Н2O - 4е → 4Н⁺ + O2(gas), φ0 = + 1.23V,
Their isolation at the anode under normal electrolysis conditions is impossible. However, a characteristic and very important feature of the anodic behavior of gold is its tendency to passivation. When gold passes into a passive state, the dissolution of the anode stops, its potential shifts to the positive side and reaches a value at which the release of chlorine gas becomes possible.
The phenomenon of passivation is extremely undesirable: at the anode, instead of the useful process of dissolving gold, a harmful process occurs - the oxidation of chlorine ions, leading to the depletion of the electrolyte in gold and poisoning of the atmosphere of the workshop.
The transition of gold to a passive state depends on the temperature of the electrolyte and especially on the concentration of hydrochloric acid in it. Thus, if in a 0.1 M solution of HAuCl4, which does not contain free hydrochloric acid, gold becomes passive at 20 °C even at very low current densities, in the same solution, but containing 1 g-eq/l of HCl, gold remains active, even at current densities of 1500A/m2.
Therefore, in order to avoid passivation of the anode and the release of chlorine on it, it is necessary to have a sufficiently high acidity and temperature of the electrolyte. In this case, the higher the anodic current density used, the more hydrochloric acid should be in the electrolyte and the higher its temperature should be. Increasing the concentration of hydrochloric acid and temperature, in addition to eliminating the passivation of gold, leads to an increase in the electrical conductivity of the electrolyte and, consequently, to a decrease in energy consumption.
Another very significant feature of gold electrolysis is that when the anode is dissolved, gold goes into solution not only in the form of the AuCl⁻4 anion, but also in the form of the AuCl⁻2 anion:
Au + 2Сl⁻ — e → АuСl2 -, φ0 =+ 1.11V.
But since the electrochemical equivalent of monovalent gold is greater than that of trivalent gold, the anodic current efficiency per trivalent gold is above 100%.
Just as this happens in the well-known process of copper electrolysis, an equilibrium is established between the AuCl⁻4 and AuCl⁻2 anions:
3АuCl⁻2 ⇄ АuCl⁻4 + 2Au + 2Сl⁻.
However, the equilibrium constant of this reaction, in contrast to the equilibrium constant of a similar reaction between Cu²⁺ and Cu⁺ ions, has a significantly smaller value.
Therefore, the concentration of AuCl⁻2 anions in the electrolyte is quite significant and is quite comparable with the concentration of AuCl4 - anions. This leads to the fact that the reduction process of AuCl⁻2 at the cathode is significantly developed.
AuCl⁻2 + e → Au + 2Cl⁻
As a result, the cathode current efficiency per trivalent gold also exceeds 100%.
Under actual conditions of electrolytic refining, the concentration of AuCl⁻2 anions formed at the anode exceeds the equilibrium value, as a result of which the equilibrium of the above disproportionation reaction shifts to the right, and part of the gold in the form of a fine powder falls into the anode slurry. Extracting gold from sludge requires additional operations, so efforts are made to prevent the formation of powdered gold. Practice has established that the transition of gold into slurry decreases with increasing current density.
And finally, the third characteristic feature of electrolytic refining of gold is that it is usually carried out with an alternating asymmetric current (Wohlville process). To do this, an alternating current generator with e. is connected in series with the direct current generator. d. s, slightly exceeding e. d.s. direct current .
The need to use asymmetric current is caused by the specific behavior of silver when dissolving the anode alloy. Being significantly more electronegative than gold, silver easily oxidizes at the anode, forming insoluble silver chloride:
Ag + Cl⁻ – e → AgCl, φ0 = + 0.22V.
If the electrolysis process is carried out using direct current, silver chloride will cover the anode with a thick crust, as a result of which the dissolution of gold will stop and chlorine gas will begin to be released at the anode. The use of asymmetric current avoids these difficulties.
When using an asymmetric current at the anode, half-periods of a positive sign alternate with shorter half-periods of a negative sign. During anodic polarization, the alloy dissolves and a film of silver chloride forms. During cathodic polarization, the AgCl film loses adhesion to the anode and falls to the bottom of the bath, turning into anode slurry. The reason for this phenomenon is, apparently, both in the partial reduction of silver chloride to metal (in the sheets in contact with the anode) and in the rapid and significant change in interfacial surface tension on the anode surface that occurs when the polarization changes.
Using an asymmetric current, it is possible to electrolyze alloys containing up to 20% Ag. In this case, Uper: Uconst. should be greater, the higher the silver content in the anodes. If the silver content in the anodes is low (less than 5-6%), then electrolysis of gold can be carried out using ordinary direct current. In this case, silver chloride easily falls into the anode slurry without forming a strong film.
In addition to silver, gold anodes usually contain copper, lead, bismuth, tellurium, iron, tin, arsenic, antimony, platinum, and palladium. The mechanism of dissolution of such a multicomponent alloy is very complex and is far from being studied. Copper, which is significantly more electronegative than gold, goes into solution, and its accumulation in the electrolyte beyond a certain limit creates the danger of a joint discharge of copper and gold. Therefore, with a high copper content in the anodes (over 2%), the electrolyte must be changed frequently. The permissible copper content in the electrolyte is 90 g/l.
Lead is even more electronegative. Dissolving at the anode first, it remains in the electrolyte in concentrations determined by the solubility of PbCl2. When the electrolyte is saturated with lead chloride, a film of solid salt PbCl2 may form on the anode, which will be deposited together with silver chloride, causing the anode to become passive. If the total content of silver and lead does not exceed 13%, the anodes are not passivated.
Bismuth, like lead, easily dissolves at the anode and its content in the alloy up to 0.3% does not cause problems. With the combined presence of 0.6% Bi, 0.9% Pb and 12% Ag in the gold alloy, the anode is passivated by a dense film, which is formed from salts of these metals. In the presence of sulfur, small amounts of lead and bismuth cause partial or even complete passivity of the anode. Thus, it has been established that alloys containing 3.6–10.1% Pb and 2.16–6.87% S are, during electrolytic dissolution, covered with a dense film of sulfur compounds, which greatly impedes dissolution. Alloys with ~13% Pb, ~3% Bi and ~12% S are completely insoluble under current.
When the alloys contain lead and bismuth sulfides, it is recommended to pre-oxidize the alloy by adding potassium permanganate to the molten metal in an amount 3-5 times higher than theoretically required for the sulfur oxidation reaction. When melting, soda is added as a coating.
Tellurium dissolves at the anode and accumulates in the electrolyte. With a significant tellurium content in the electrolyte, the quality of the cathode deposits deteriorates.
Iron is a harmful impurity during electrolysis. Passing into solution in the form of Fe²⁺ ions, it reduces gold from the electrolyte and increases its content in the sludge.
Tin, arsenic and antimony, being in the alloy in small quantities (up to 0.05%), dissolve well and do not cause difficulties. Platinum and palladium dissolve at the anode, forming chloroplatinic acid and palladium chloride.
Since the standard potentials of these metals are close to the standard potential of gold:
PtCl²6⁻ + 4е → 6Сl⁻ + Pt, φ0 = + 0.73V;
Pd²⁺ + 2е → Pd, φ0 = + 0.99V,
then, if there is excessive accumulation in the solution, their deposition can begin at the cathode together with gold. The maximum permissible concentration of platinum in the electrolyte is 50 and palladium 15 g/l. Ruthenium, rhodium, osmium and iridium (if present in the anodes) completely go into solution.
Electrolysis of gold is carried out in small baths made of porcelain or vinyl plastic with a capacity of 20-65 liters.
In domestic practice, porcelain baths with a capacity of 25 liters are used. Gold plate 0.1–0.25 mm thick, produced by rolling pure electrolytic gold, is used as cathodes. To give the cathodes rigidity, they are corrugated on a special press. 18 cathodes (not shown in the figure) are suspended in the baths on six rods (three cathodes in a row) and 15 anodes on five rods (three anodes in a row). The mass of one anode is approximately 2 kg. The anodes are suspended from the rods using gold ribbons fused into the metal when the anodes are cast. To maintain the required temperature of the electrolyte, the baths are installed in water baths. The electrolyte is mixed with compressed air supplied to the baths through glass tubes. Since electrolysis produces chlorine, the baths are placed in a special fume hood. The current is supplied outside the cabinet via copper bars, and inside - silver bars as they are more resistant to chlorine atmosphere. Rods for hanging electrodes are made from silver.
The electrolyte contains 70–200 g/l Au and 40–100 g/l HCl. Electrolyte temperature 50-60 °C. Electrolysis is carried out with an asymmetric current with a density of 600-1500 A/m². The strength of alternating current is usually 10% higher than direct current. The voltage across the bath is 0.5-1 V. Gold is deposited on the cathode in the form of a dense, shiny sediment. The cathodes are unloaded 3-4 times a day, depending on the current density used.
Cathode gold is washed with hot water, cleaned with brushes, treated with hydrochloric acid or ammonia (to dissolve stray particles of silver chloride), washed again with water, dried and melted in an induction furnace into ingots. The purity of cathode gold is 999.8-999.9 samples. The main impurities in it are silver, copper, iron.
The anode slurry is unloaded from the baths and washed with water to remove the electrolyte. Rinse water is used to top up baths. The sludge is loaded into a silver mesh drum placed in a water-filled bath. As the drum rotates, silver chloride is washed through the holes into the bath, and larger particles of gold anode scrap and cathode gold dendrites remain in the drum. The gold residues are dried and returned to the smelting for the anodes. Silver chloride is reduced with iron scrap or powder in a hydrochloric acid environment, washed with water and melted into anodes for silver electrolysis.
The yield of anodic scrap during gold electrolysis depends on the purity of the anodes and ranges from 10 to 20% of the mass of the original anodes. Just like sludge, anode residues are washed in a mesh drum to remove silver chloride and electrolyte, dried and melted into anodes.
During the electrolysis process, the electrolyte becomes enriched in impurities and depleted in gold. When working with a dirty electrolyte, contamination of the cathode deposits may occur due to coprecipitation of impurities. In addition, when the electrolyte is contaminated, dendrites begin to grow at the cathode, which leads to short-circuiting of the electrodes, and at the anode, crystallization of salts begins, leading to passivation of the anodes. The electrolyte is unfit for further use if the concentration of gold in it is below 100 g/l, and the concentration of impurities is above the following limits, g/l:
90 Cu, 50 Pt, 15 Pd, 1.5 Pb, 4 Te. 2 Fe.
For processing, the spent electrolyte is poured into special baths, where most of the gold is extracted from it by electrolysis with insoluble anodes. The cathodes are thin gold plates, the anodes are made of graphite. The process is carried out using direct current with a density of 200-500 A/m². Platinum and palladium are precipitated from the resulting solution with ammonium chloride, and then the remaining gold is precipitated using a solution of ferric chloride. Copper is cemented with iron.
Other methods for processing spent electrolyte are also possible, in particular, using ion exchange resins.
Fresh electrolyte is prepared by electrochemical dissolution of relatively pure gold alloys, most often obtained by processing the anode sludge of silver electrolysis. Dissolution is carried out in special round baths equipped with diaphragms! made of porous porcelain, clay or ion exchange film. 6-8 anodes are hung into the diaphragm and hydrochloric acid with a density of 1.19, diluted with water in a ratio of 3:1, is poured. Cathodes—thin plates of gold or graphite—are suspended on either side of the diaphragm. More dilute (1:3) hydrochloric acid is poured into the cathode space. When a direct current is passed through, gold dissolves at the anode and hydrogen is reduced at the cathode. The overall reaction is expressed by the following equation:
2Au + 6Н⁺ + 8Сl⁻ = 2АuСl⁻4 + 3Н2.
The process is carried out using direct current with a density of 800-2000 A/m². The voltage on the bath for a diaphragm made of ion-exchange film is up to 4 V, for a diaphragm made of clay - up to 14 V. The electrolyte temperature is 60-90 ° C.
The resulting solution contains 200-300 g/l Au and 45-80 g/l hydrochloric acid. It is diluted with water and poured into the main electrolysis baths. Fresh electrolyte can also be prepared by dissolving cathode gold in hydrochloric acid while passing chlorine gas.
The advantage of the process of electrolytic refining of gold is not only the possibility of obtaining high-purity metal that meets the requirements of modern technology, but also the associated recovery of platinum metals lost during refining by chlorination. In South Africa, part of the gold that has been refined by chlorination is subjected to electrolytic refining. In this case, those batches of gold that contain platinum metals are preferably sent for electrolysis.
You are reading an article on the topic Electrolytic refining of gold
How to extract gold metal from memory cards, microcircuits and radio components
Technical waste like transistors and microcircuits actually contains gold, which covers contacts and connectors with a thin layer. The bodies of the parts are coated with kovar - an alloy of iron with nickel and cobalt - so the extraction of gold involves dissolving kovar with nitric acid (in which gold, as we already know, does not dissolve).

From the resulting material, the remains of the kovar are selected with a powerful magnet (it is better to use neodymium).
The process of refining gold at home
If you decide to conduct the experiment at home, you need to prepare. First of all, think about safety precautions: you will need a mask to protect against fumes, gloves and caution. You can experiment only in a well-ventilated area, where children and animals do not have access.
Necessary raw materials, equipment and reagents
Let's imagine that we were able to get everything we needed for work and decided to try. Let's get started with quartering: let's bring the gold sample to 200-250, diluting the starting material with copper, and then dissolve the alloy. Zinc would dissolve faster than copper, but the alloy with it is very fragile, and copper is easier to control for a beginner.
For refining we will need:
- gold scrap, the standard of which will be increased;
- two crucibles (you can buy them or make them yourself from fireclay clay and fire them);
- 999 fine copper (it is convenient to buy granulated copper at the rate of N×3–m, where N is the approximate mass of gold in our scrap, and m is the approximate mass of the alloy);
- nitric acid (also sold, its amount should be 10 times the total amount of metal);
- hydrochloric acid;
- borax (antiseptic sodium tetraborate, available at the pharmacy);
- melting burner;
- a wooden (birch, aspen) stick for stirring the melt (some jewelers recommend using graphite pencil rods);
- long tweezers;
- gauze;
- a cap with a hole for covering the crucible;
- electric stove;
- glass fireproof flask for boiling.
Preparation
Let's say we have 20 g of 585-carat jewelry gold, that is, 11.7 g of pure metal and 8.3 g of impurities are alloyed. For quartering you will need copper in the amount of 26.8 g (11.7 × 3–8.3).
- We chop the scrap - for example, using wire cutters.
- We dry and heat the crucibles, otherwise there is a risk that they will burst.
- Add copper granules to the original gold with tweezers and stir until it melts evenly, without stopping heating. We take each new piece after the previous one has melted.
- We roll out or granulate the mass by pouring it into water in a thin stream (the zinc alloy can be crushed even in a mortar).
Affination process
Important: this part of the process is carried out only in fresh air, otherwise it is life-threatening! The ideal option is a summer cottage that has an extension cord to connect to the electric stove.
Add 60–70 ml of nitric acid to the flask with crushed metal. As the reaction subsides, add another 40–50 ml so that the interaction resumes (the total volume of acid should not exceed 200 ml). We repeat the procedure 2-3 times, after which we bring the flask to a boil.
We wash the precipitate with clean water, adding it to the flask until the mixture becomes transparent, and carefully draining it. Then, with a small amount of water, sprinkled generously with borax, we place the gold in a clean crucible (it’s convenient to use gauze for this - you’ll get a “knot” of gold flakes).
The crucible with wrapped gold, sprinkled with borax, covered with a cap, is kept on the fire until the gauze wears out. Having made sure that the mixture of borax and gold has become more or less homogeneous, we proceed to melting. Sprinkle the molten metal with borax again.
The refining process can be considered complete when the gold that begins to harden does not become cloudy, but remains shiny. Until this happens, we continue to melt it and sprinkle it with borax.
Pure gold melts very beautifully. You can see how professional jewelers melt it in the video:
Some concepts and definitions
The terms “affinage” and “refining” come from the French verb affiner (to purify) and describe in metallurgy the sequence of technological processes for obtaining precious metals of high purity. It is known that the popular 585 gold has only 58.5% of the noble source, and the rest is additives that give jewelry samples the necessary qualities (strength, weight, color).
Refining precious metals involves purifying everything unnecessary. Various methods of targeted physical and chemical influence make it possible not only to separate substances, but also to remove contaminants from the composition. The raw materials for refining are various materials:

- jewelry or technological scrap;
- gold-containing concentrates from mines;
- grains of native schlich gold;
- sludge from electrolysis coating shops of zinc and silver, nickel and copper;
- silver foam from metallurgical lead production plants.
The preparation of any raw material consists of crushing, roasting and fusion. For example, radio-electronic parts are first dismantled and the housing and switching part, which does not contain precious metals, are separated. This allows you to reduce the number of environmental problems during processing and obtain a gold purity of up to 996.5 (99.65%), and silver – up to 999 (99.90%).
All technologies can be roughly divided into three conditional groups:
- Wet. They are based on chemical transformations.
- Dry ones that use chlorine vapor treatment.
- Electrolysis in special baths.
Some methods require expensive equipment and technology and are applicable only in specialized metallurgical enterprises, while others are quite easily performed by craftsmen. Chemical refining is suitable for both a jewelry workshop and a home laboratory, but in the latter case, work cannot be started without a fume hood and the use of personal protective equipment. Among the instruments and chemical utensils you will definitely need to purchase:

- crucible for heating, roasting, burning or melting materials over an open fire;
- durable tweezers;
- steel knitting needle;
- heat-resistant glass flask;
- electric stove for heating solutions;
- burner for melting metals;
- Filtration equipment: container, gauze, paper.
In industry, a “dry” method is used for refining gold and silver: contaminated melts are treated with chlorine, and volatile reaction products (impurity chlorides) are captured by special equipment.
Electrolysis is widely used, when the purified substance is collected at the cathode, and purification by cupellation, based on the properties of lead to react with oxygen in the air, and then evaporate and deposit on the walls of the furnace.